Effects of Estetrol on Migration and Invasion in T47-D Breast Cancer Cells through the Actin Cytoskeleton
- PMID: 24904530
- PMCID: PMC4033260
- DOI: 10.3389/fendo.2014.00080
Effects of Estetrol on Migration and Invasion in T47-D Breast Cancer Cells through the Actin Cytoskeleton
Abstract
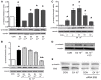
Estetrol (E4) is a natural human estrogen present at high concentrations during pregnancy. Due to its high oral bioavailability and long plasma half-life, E4 is particularly suitable for therapeutic applications. E4 acts as a selective estrogen receptor (ER) modulator, exerting estrogenic actions on the endometrium or the central nervous system, while antagonizing the actions of estradiol in the breast. We tested the effects of E4 on its own or in the presence of 17β-estradiol (E2) on T47-D ER+ breast cancer cell migration and invasion of three-dimensional matrices. E4 administration to T47-D cells weakly stimulated migration and invasion. However, E4 decreased the extent of movement and invasion induced by E2. Breast cancer cell movement requires a remodeling of the actin cytoskeleton. During exposure to E4, a weak, concentration-dependent, re-distribution of actin fibers toward the cell membrane was observed. However, when E4 was added to E2, an inhibition of actin remodeling induced by E2 was seen. Estrogens stimulate ER+ breast cancer cell movement through the ezrin-radixin-moesin family of actin regulatory proteins, inducing actin and cell membrane remodeling. E4 was a weak inducer of moesin phosphorylation on Thr(558), which accounts for its functional activation. In co-treatment with E2, E4 blocked the activation of this actin controller in a concentration-related fashion. These effects were obtained through recruitment of estrogen receptor-α. In conclusion, E4 acted as a weak estrogen on breast cancer cell cytoskeleton remodeling and movement. However, when E2 was present, E4 counteracted the stimulatory actions of E2. This contributes to the emerging hypothesis that E4 may be a naturally occurring ER modulator in the breast.
Keywords: actin cytoskeleton; breast cancer; cancer progression; estetrol; estrogen.
Figures



References
-
- Ricketts D, Turnbull L, Ryall G, Bakhshi R, Rawson NS, Gazet JC, et al. Estrogen and progesterone receptors in the normal female breast. Cancer Res (1991) 51(7):1817–22 - PubMed
-
- Kelsey JL, Gammon MD, John EM. Reproductive factors and breast cancer. Epidemiol Rev (1993) 15(1):36–47 - PubMed
-
- Hagen AA, Barr M, Diczfalusy E. Metabolism of 17-beta-oestradiol-4-14-C in early infancy. Acta Endocrinol (1965) 49:207–20 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

