The activity of Nef on HIV-1 infectivity
- PMID: 24904546
- PMCID: PMC4033043
- DOI: 10.3389/fmicb.2014.00232
The activity of Nef on HIV-1 infectivity
Abstract
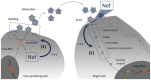
The replication and pathogenicity of lentiviruses is crucially modulated by "auxiliary proteins" which are expressed in addition to the canonical retroviral ORFs gag, pol, and env. Strategies to inhibit the activity of such proteins are often sought and proposed as possible additions to increase efficacy of the traditional antiretroviral therapy. This requires the acquisition of an in-depth knowledge of the molecular mechanisms underlying their function. The Nef auxiliary protein is expressed uniquely by primate lentiviruses and plays an important role in virus replication in vivo and in the onset of AIDS. Among its several activities Nef enhances the intrinsic infectivity of progeny virions through a mechanism which remains today enigmatic. Here we review the current knowledge surrounding such activity and we discuss its possible role in HIV biology.
Keywords: AIDS; HIV; Nef; auxiliary proteins; retrovirus infectivity.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

