Antibacterial activities of bacteriocins: application in foods and pharmaceuticals
- PMID: 24904554
- PMCID: PMC4033612
- DOI: 10.3389/fmicb.2014.00241
Antibacterial activities of bacteriocins: application in foods and pharmaceuticals
Erratum in
-
Corrigendum: Antibacterial activities of bacteriocins: application in foods and pharmaceuticals.Front Microbiol. 2014 Dec 5;5:683. doi: 10.3389/fmicb.2014.00683. eCollection 2014. Front Microbiol. 2014. PMID: 25544112 Free PMC article.
Abstract
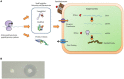
Bacteriocins are a kind of ribosomal synthesized antimicrobial peptides produced by bacteria, which can kill or inhibit bacterial strains closely-related or non-related to produced bacteria, but will not harm the bacteria themselves by specific immunity proteins. Bacteriocins become one of the weapons against microorganisms due to the specific characteristics of large diversity of structure and function, natural resource, and being stable to heat. Many recent studies have purified and identified bacteriocins for application in food technology, which aims to extend food preservation time, treat pathogen disease and cancer therapy, and maintain human health. Therefore, bacteriocins may become a potential drug candidate for replacing antibiotics in order to treat multiple drugs resistance pathogens in the future. This review article summarizes different types of bacteriocins from bacteria. The latter half of this review focuses on the potential applications in food science and pharmaceutical industry.
Keywords: bacteriocin; cancer treatment; food; natural product; protein.
Figures

References
-
- Babasaki K., Takao T., Shimonishi Y., Kurahashi K. (1985). Subtilosin A, a new antibiotic peptide produced by Bacillus subtilis 168: isolation, structural analysis, and biogenesis. J. Bacteriol. 98, 585–603 - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

