Cellular unfolded protein response against viruses used in gene therapy
- PMID: 24904562
- PMCID: PMC4033601
- DOI: 10.3389/fmicb.2014.00250
Cellular unfolded protein response against viruses used in gene therapy
Abstract
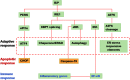
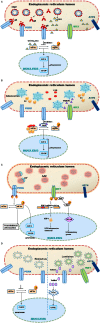
Viruses are excellent vehicles for gene therapy due to their natural ability to infect and deliver the cargo to specific tissues with high efficiency. Although such vectors are usually "gutted" and are replication defective, they are subjected to clearance by the host cells by immune recognition and destruction. Unfolded protein response (UPR) is a naturally evolved cyto-protective signaling pathway which is triggered due to endoplasmic reticulum (ER) stress caused by accumulation of unfolded/misfolded proteins in its lumen. The UPR signaling consists of three signaling pathways, namely PKR-like ER kinase, activating transcription factor 6, and inositol-requiring protein-1. Once activated, UPR triggers the production of ER molecular chaperones and stress response proteins to help reduce the protein load within the ER. This occurs by degradation of the misfolded proteins and ensues in the arrest of protein translation machinery. If the burden of protein load in ER is beyond its processing capacity, UPR can activate pro-apoptotic pathways or autophagy leading to cell death. Viruses are naturally evolved in hijacking the host cellular translation machinery to generate a large amount of proteins. This phenomenon disrupts ER homeostasis and leads to ER stress. Alternatively, in the case of gutted vectors used in gene therapy, the excess load of recombinant vectors administered and encountered by the cell can trigger UPR. Thus, in the context of gene therapy, UPR becomes a major roadblock that can potentially trigger inflammatory responses against the vectors and reduce the efficiency of gene transfer.
Keywords: ER-homeostasis; ER-stress; UPR; chaperones; gene therapy; viral vectors.
Figures
Similar articles
-
Virus-induced ER stress and the unfolded protein response.Front Plant Sci. 2012 Dec 28;3:293. doi: 10.3389/fpls.2012.00293. eCollection 2012. Front Plant Sci. 2012. PMID: 23293645 Free PMC article.
-
The Human Cytomegalovirus Endoplasmic Reticulum-Resident Glycoprotein UL148 Activates the Unfolded Protein Response.J Virol. 2018 Sep 26;92(20):e00896-18. doi: 10.1128/JVI.00896-18. Print 2018 Oct 15. J Virol. 2018. PMID: 30045994 Free PMC article.
-
Thiopurines activate an antiviral unfolded protein response that blocks influenza A virus glycoprotein accumulation.J Virol. 2021 May 10;95(11):e00453-21. doi: 10.1128/JVI.00453-21. Epub 2021 Mar 24. J Virol. 2021. PMID: 33762409 Free PMC article.
-
Endoplasmic reticulum proteostasis: a key checkpoint in cancer.Am J Physiol Cell Physiol. 2017 Feb 1;312(2):C93-C102. doi: 10.1152/ajpcell.00266.2016. Epub 2016 Nov 16. Am J Physiol Cell Physiol. 2017. PMID: 27856431 Free PMC article. Review.
-
ER Stress and Unfolded Protein Response in Leukemia: Friend, Foe, or Both?Biomolecules. 2021 Jan 30;11(2):199. doi: 10.3390/biom11020199. Biomolecules. 2021. PMID: 33573353 Free PMC article. Review.
Cited by
-
FVIII activity following FVIII protein infusion or FVIII gene transfer predicts the bleeding risk in hemophilia A rats.J Thromb Haemost. 2020 Jul;18(7):1586-1597. doi: 10.1111/jth.14804. Epub 2020 Apr 16. J Thromb Haemost. 2020. PMID: 32196903 Free PMC article.
-
Virus and tumor microenvironment induced ER stress and unfolded protein response: from complexity to therapeutics.Oncotarget. 2018 Aug 7;9(61):31920-31936. doi: 10.18632/oncotarget.25886. eCollection 2018 Aug 7. Oncotarget. 2018. PMID: 30159133 Free PMC article. Review.
-
The unfolded protein response in virus infections.Front Microbiol. 2014 Sep 30;5:518. doi: 10.3389/fmicb.2014.00518. eCollection 2014. Front Microbiol. 2014. PMID: 25324837 Free PMC article. No abstract available.
-
Optimized Parameters for Transducing the Locus Coeruleus Using Canine Adenovirus Type 2 (CAV2) Vector in Rats for Chemogenetic Modulation Research.Front Neurosci. 2021 Apr 13;15:663337. doi: 10.3389/fnins.2021.663337. eCollection 2021. Front Neurosci. 2021. PMID: 33927593 Free PMC article.
-
Optimized AAV rh.10 Vectors That Partially Evade Neutralizing Antibodies during Hepatic Gene Transfer.Front Pharmacol. 2017 Jul 17;8:441. doi: 10.3389/fphar.2017.00441. eCollection 2017. Front Pharmacol. 2017. PMID: 28769791 Free PMC article.
References
-
- Amalfitano A., McVie-Wylie A. J., Hu H., Dawson T. L., Raben N., Plotz P., et al. (1999). Systemic correction of the muscle disorder glycogen storage disease type II after hepatic targeting of a modified adenovirus vector encoding human acid-alpha-glucosidase. Proc. Natl. Acad. Sci. U.S.A. 96, 8861–8866 10.1073/pnas.96.16.8861 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

