The role of placental tryptophan catabolism
- PMID: 24904580
- PMCID: PMC4032907
- DOI: 10.3389/fimmu.2014.00230
The role of placental tryptophan catabolism
Abstract
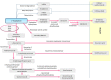
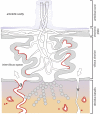
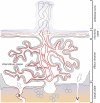
This review discusses the mechanisms and consequences of degradation of tryptophan (Trp) in the placenta, focusing mainly on the role of indoleamine 2,3-dioxygenase-1 (IDO1), one of three enzymes catalyzing the first step of the kynurenine pathway of Trp degradation. IDO1 has been implicated in regulation of feto-maternal tolerance in the mouse. Local depletion of Trp and/or the presence of metabolites of the kynurenine pathway mediate immunoregulation and exert antimicrobial functions. In addition to the decidual glandular epithelium, IDO1 is localized in the vascular endothelium of the villous chorion and also in the endothelium of spiral arteries of the decidua. Possible consequences of IDO1-mediated catabolism of Trp in the endothelium encompass antimicrobial activity and immunosuppression, as well as relaxation of the placental vasotonus, thereby contributing to placental perfusion and growth of both placenta and fetus. It remains to be evaluated whether other enzymes mediating Trp oxidation, such as indoleamine 2,3-dioxygenase-2, Trp 2,3-dioxygenase, and Trp hydroxylase-1 are of relevance to the biology of the placenta.
Keywords: fetal growth restriction; feto-maternal tolerance; immunoregulation; intrauterine growth restriction; placenta; preeclampsia; pregnancy; vasotonus.
Figures
References
-
- Food and Nutrition Board. (1974). Recommended Dietary Allowances, 8th ed Washington DC: National Academy of Sciences; .
-
- Ikeda M, Tsuji H, Nakamura S, Ichiyama A, Nishizuka Y, Hayaishi O. Studies on the biosynthesis of nicotinamide adenine dinucleotide. Ii. A role of picolinic carboxylase in the biosynthesis of nicotinamide adenine dinucleotide from tryptophan in mammals. J Biol Chem (1965) 240:1395–401 - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

