Cancer epigenetics: tumor heterogeneity, plasticity of stem-like states, and drug resistance
- PMID: 24905005
- PMCID: PMC4103691
- DOI: 10.1016/j.molcel.2014.05.015
Cancer epigenetics: tumor heterogeneity, plasticity of stem-like states, and drug resistance
Abstract
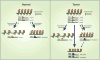
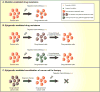
The existence of subpopulations of cells in cancers with increased tumor-initiating capacities and self-renewal potential, often termed "cancer stem cells," is a much discussed and key area of cancer biology. Such cellular heterogeneity is very important because of its impact on therapy and especially states of treatment resistance. A major question is whether there is plasticity for evolution of these cell states during tumorigenesis that can involve movement between cell populations in a reversible fashion. In this review, we discuss the possible role of epigenetic abnormalities as well as genetic alterations in such dynamics and in the creation of cellular heterogeneity in cancers of all types.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures


References
-
- Ahuja N, Li Q, Mohan AL, Baylin SB, Issa JP. Aging and DNA methylation in colorectal mucosa and cancer. Cancer Res. 1998;58:5489–5494. - PubMed
-
- Allis CD, Jenuwein T, Reinberg D. Epigenetics. 1st. Cold Spring Harbor Laboratory Press; 2008.
-
- Amary MF, Bacsi K, Maggiani F, Damato S, Halai D, Berisha F, Pollock R, O'Donnell P, Grigoriadis A, Diss T, et al. IDH1 and IDH2 mutations are frequent events in central chondrosarcoma and central and periosteal chondromas but not in other mesenchymal tumours. J Pathol. 2011;224:334–343. - PubMed
-
- Anderson K, Lutz C, van Delft FW, Bateman CM, Guo Y, Colman SM, Kempski H, Moorman AV, Titley I, Swansbury J, et al. Genetic variegation of clonal architecture and propagating cells in leukaemia. Nature. 2011;469:356–361. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

