Testicular differentiation factor SF-1 is required for human spleen development
- PMID: 24905461
- PMCID: PMC4001552
- DOI: 10.1172/JCI73186
Testicular differentiation factor SF-1 is required for human spleen development
Abstract
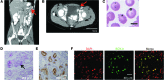
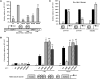
The transcription factor steroidogenic factor 1 (SF-1; also known as NR5A1) is a crucial mediator of both steroidogenic and nonsteroidogenic tissue differentiation. Mutations within SF1 underlie different disorders of sexual development (DSD), including sex reversal, spermatogenic failure, ovarian insufficiency, and adrenocortical deficiency. Here, we identified a recessive mutation within SF1 that resulted in a substitution of arginine to glutamine at codon 103 (R103Q) in a child with both severe 46,XY-DSD and asplenia. The R103Q mutation decreased SF-1 transactivation of TLX1, a transcription factor that has been shown to be essential for murine spleen development. Additionally, the SF1 R103Q mutation impaired activation of steroidogenic genes, without affecting synergistic SF-1 and sex-determining region Y (SRY) coactivation of the testis development gene SOX9. Together, our data provide evidence that SF-1 is required for spleen development in humans via transactivation of TLX1 and that mutations that only impair steroidogenesis, without altering the SF1/SRY transactivation of SOX9, can lead to 46,XY-DSD.
Figures

References
-
- Achermann JC, et al. Gonadal determination and adrenal development are regulated by the orphan nuclear receptor steroidogenic factor-1, in a dose-dependent manner. J Clin Endocrinol Metab. 2002;87(4):1829–1833. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

