Epidermal growth factor inhibits transforming growth factor-β-induced fibrogenic differentiation marker expression through ERK activation
- PMID: 24905473
- PMCID: PMC4130781
- DOI: 10.1016/j.cellsig.2014.05.018
Epidermal growth factor inhibits transforming growth factor-β-induced fibrogenic differentiation marker expression through ERK activation
Abstract
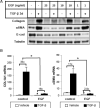
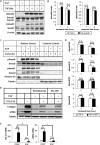
Transforming growth factor-β (TGF-β) signaling plays an important and complex role in renal fibrogenesis. The seemingly simple TGF-β/Smad cascade is intensively regulated at several levels, including crosstalk with other signaling pathways. Epidermal growth factor (EGF) is a potent mitogen for epithelial cells and is elevated in diseased kidneys. In this study, we examined its effect on TGF-β-induced fibrotic changes in human proximal tubular epithelial cells. Simultaneous treatment with EGF specifically inhibited basal and TGF-β-induced type-I collagen and α-smooth muscle actin (αSMA) expression at both mRNA and protein levels. These effects were prevented by inhibition of either the EGF receptor kinase or its downstream MEK kinase but not by blockade of either the JNK or PI3K pathway. Overexpression of a constitutively active MEK1 construct mimicked the inhibitory effect of EGF. Further, EGF suppressed Smad transcriptional activities, as shown by reduced activation of ARE-luc and SBE-luc. Both reductions were prevented by MEK inhibition. However, EGF did not block Smad2 or Smad3 phosphorylation by TGF-β, or Smad2/3 nuclear import. Finally EGF induced the phosphorylation and expression of TGIF, a known TGF-β/Smad repressor. Both the phosphorylation and the induction were blocked by a MEK inhibitor. Overexpression of TGIF abolished TGF-β-induced αSMA promoter activity. Together these results suggest that EGF inhibits two TGF-β-stimulated markers of EMT through EGF receptor tyrosine kinase and downstream ERK activation, but not through PI3K or JNK. The inhibition results from effector mechanisms downstream of Smads, and most likely involves the transcriptional repressor, TGIF.
Keywords: EGF; EMT; ERK; Fibrosis; Smad2/3; TGF-β.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures





References
-
- Burns WC, Thomas MC. The molecular mediators of type 2 epithelial to mesenchymal transition (EMT) and their role in renal pathophysiology. Expert Rev Mol Med. 2010;12:e17. - PubMed
-
- Poncelet AC, Schnaper HW. Sp1 and Smad proteins cooperate to mediate transforming growth factor-beta 1-induced alpha 2(I) collagen expression in human glomerular mesangial cells. The Journal of biological chemistry. 2001;276(10):6983–92. - PubMed
-
- Hu B, Wu Z, Phan SH. Smad3 mediates transforming growth factor-beta-induced alpha-smooth muscle actin expression. Am J Respir Cell Mol Biol. 2003;29(3 Pt 1):397–404. - PubMed
-
- Barrientos S, et al. Growth factors and cytokines in wound healing. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2008;16(5):585–601. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

