Kinetics and prediction of HBsAg loss during long-term therapy with nucleos(t)ide analogues of different potency in patients with chronic hepatitis B
- PMID: 24905586
- PMCID: PMC4048200
- DOI: 10.1371/journal.pone.0098476
Kinetics and prediction of HBsAg loss during long-term therapy with nucleos(t)ide analogues of different potency in patients with chronic hepatitis B
Abstract
Background & aims: About 350-400 million people are infected with hepatitis B virus (HBV) chronically and 1 million people die of hepatitis B virus (HBV)-related liver diseases. Nucleos(t)ide analogues (NAs) have been used for the treatment against HBV. However, few studies have investigated the long-term effects of different nucleos(t)ide analogues on levels of hepatitis B surface antigen (HBsAg) in patients with chronic hepatitis B (CHB). The aims of this study were to measure the magnitude of HBsAg reduction by long-term monotherapy with adefovir dipivoxil (ADV) and entecavir (ETV), to compare HBsAg reduction between the two drugs of different potency and to predict the expected time needed to achieve HBsAg loss.
Methods: We retrospectively evaluated the kinetics of HBsAg in 67 patients with CHB who all exhibited persistent viral suppression. These patients were treated with ADV or ETV for at least 6 years. HBV genotype was determined at baseline. Liver biochemistry, HBV serological markers, serum HBV DNA and HBsAg titers were determined at baseline, half year and yearly from year 1 to 6.
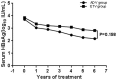
Results: Serum HBsAg titers after treatment with ADV or ETV were significantly lower than the baseline titers (P<0.05). HBsAg reduction rate of patients treated with ETV (0.11 log10 IU/mL/ year) was higher than that treated with ADV (0.10 log10 IU/mL/year), and the calculated expected time to HBsAg loss for patients treated with ETV (approximate 24.99 years) was shorter than that with ADV (approximate 30.33 years), but there was no statistically significant difference between two groups (P>0.05).
Conclusion: Serum HBsAg titers gradually decreased during long-term treatment with either ADV or ETV. It appears that the potency of ADV on HBsAg reduction is close to that of ETV, as long as patients have achieved persistent viral suppression.
Conflict of interest statement
Figures


References
-
- Ganem D, Prince AM (2004) Hepatitis B virus infection-natural history and clinical consequences. N Engl J Med 350: 1118–29. - PubMed
-
- Woo G, Tomlinson G, Nishikawa Y, Kowgier M, Sherman M, et al. (2010) Tenofovir and entecavir are the most effective antiviral agents for chronic hepatitis B: a systematic review and Bayesian meta-analyses. Gastroenterology 139: 1218–29. - PubMed
-
- Feld JJ, Wong DK, Heathcote EJ (2009) Endpoints of therapy in chronic hepatitis B. Hepatology. 49: S96–S102. - PubMed
-
- Zoulim F (2005) New insight on hepatitis B virus persistence from the study of intrahepatic viral cccDNA. J Hepatol 42: 302–8. - PubMed
-
- Werle-Lapostolle B, Bowden S, Locarnini S, Wursthorn K, Petersen J, et al. (2004) Persistence of cccDNA during the natural history of chronic hepatitis B and decline during adefovir dipivoxil therapy. Gastroenterology 126: 1750–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

