Mechanosensing at the vascular interface
- PMID: 24905872
- PMCID: PMC4450720
- DOI: 10.1146/annurev-bioeng-071813-104908
Mechanosensing at the vascular interface
Abstract
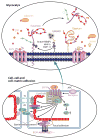
Mammals are endowed with a complex set of mechanisms that sense mechanical forces imparted by blood flow to endothelial cells (ECs), smooth muscle cells, and circulating blood cells to elicit biochemical responses through a process referred to as mechanotransduction. These biochemical responses are critical for a host of other responses, including regulation of blood pressure, control of vascular permeability for maintaining adequate perfusion of tissues, and control of leukocyte recruitment during immunosurveillance and inflammation. This review focuses on the role of the endothelial surface proteoglycan/glycoprotein layer-the glycocalyx (GCX)-that lines all blood vessel walls and is an agent in mechanotransduction and the modulation of blood cell interactions with the EC surface. We first discuss the biochemical composition and ultrastructure of the GCX, highlighting recent developments that reveal gaps in our understanding of the relationship between composition and spatial organization. We then consider the roles of the GCX in mechanotransduction and in vascular permeability control and review the prominent interaction of plasma-borne sphingosine-1 phosphate (S1P), which has been shown to regulate both the composition of the GCX and the endothelial junctions. Finally, we consider the association of GCX degradation with inflammation and vascular disease and end with a final section on future research directions.
Keywords: endothelium; glycocalyx; glycoprotein; proteoglycan; red cell; shear stress; sphingosine-1 phosphate; vascular disease; vascular permeability; white cell.
Figures

References
-
- Nerem RM, Levesque MJ, Cornhill JF. Vascular endothelial morphology as an indicator of the pattern of blood flow. J Biomech Eng. 1981;103:172–76. - PubMed
-
- Kuchan MJ, Frangos JA. Role of calcium and calmodulin in flow-induced nitric oxide production in endothelial cells. Am J Physiol. 1994;266:C628–36. - PubMed
-
- Frangos JA, Eskin SG, McIntire LV, Ives CL. Flow effects on prostacyclin production by cultured human endothelial cells. Science. 1985;227:1477–79. - PubMed
-
- Mo M, Eskin SG, Schilling WP. Flow-induced changes in Ca2+ signaling of vascular endothelial cells: effect of shear stress and ATP. Am J Physiol. 1991;260:H1698–707. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL094889/HL/NHLBI NIH HHS/United States
- R37 HL028607/HL/NHLBI NIH HHS/United States
- R01 AI047294/AI/NIAID NIH HHS/United States
- HL 28607/HL/NHLBI NIH HHS/United States
- R01 HL044485/HL/NHLBI NIH HHS/United States
- HL94889/HL/NHLBI NIH HHS/United States
- R01 HL057093/HL/NHLBI NIH HHS/United States
- HL57093/HL/NHLBI NIH HHS/United States
- R01 HL028607/HL/NHLBI NIH HHS/United States
- R01 HL082689/HL/NHLBI NIH HHS/United States
- HL44485/HL/NHLBI NIH HHS/United States
- AI47294/AI/NIAID NIH HHS/United States
- HL082689/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

