3D biofabrication strategies for tissue engineering and regenerative medicine
- PMID: 24905875
- PMCID: PMC4131759
- DOI: 10.1146/annurev-bioeng-071813-105155
3D biofabrication strategies for tissue engineering and regenerative medicine
Abstract
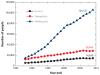
Over the past several decades, there has been an ever-increasing demand for organ transplants. However, there is a severe shortage of donor organs, and as a result of the increasing demand, the gap between supply and demand continues to widen. A potential solution to this problem is to grow or fabricate organs using biomaterial scaffolds and a person's own cells. Although the realization of this solution has been limited, the development of new biofabrication approaches has made it more realistic. This review provides an overview of natural and synthetic biomaterials that have been used for organ/tissue development. It then discusses past and current biofabrication techniques, with a brief explanation of the state of the art. Finally, the review highlights the need for combining vascularization strategies with current biofabrication techniques. Given the multitude of applications of biofabrication technologies, from organ/tissue development to drug discovery/screening to development of complex in vitro models of human diseases, these manufacturing technologies can have a significant impact on the future of medicine and health care.
Keywords: bioprinting; hydrogels; photolithography; scaffolds; stem cells; vascularization.
Figures





References
-
- US Dep. Health Hum. Serv. Donate the Gift of Life Statistics and Figures. US Dep Health Hum Serv; Washington, DC: 2014. The need is real: data. retrieved February 26, 2014 http://www.organdonor.gov/about/data.html.
-
- Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–26. - PubMed
-
- Nerem RM. Cellular engineering. Ann Biomed Eng. 1991;19:529–45. - PubMed
-
- Compton CC, Butler CE, Yannas IV, Warland G, Orgill DP. Organized skin structure is regenerated in vivo from collagen-GAG matrices seeded with autologous keratinocytes. J Investig Dermatol. 1998;110:908–16. - PubMed
-
- Vunjak-Novakovic G, Obradovic B, Martin I, Bursac PM, Langer R, Freed LE. Dynamic cell seeding of polymer scaffolds for cartilage tissue engineering. Biotechnol Prog. 1998;14:193–202. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

