Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis
- PMID: 24906147
- PMCID: PMC4131287
- DOI: 10.1016/j.cell.2014.03.065
Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis
Abstract
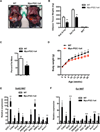
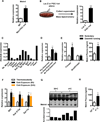
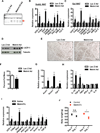
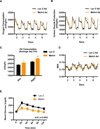
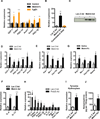
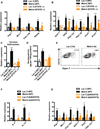
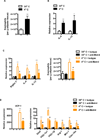
Exercise training benefits many organ systems and offers protection against metabolic disorders such as obesity and diabetes. Using the recently identified isoform of PGC1-α (PGC1-α4) as a discovery tool, we report the identification of meteorin-like (Metrnl), a circulating factor that is induced in muscle after exercise and in adipose tissue upon cold exposure. Increasing circulating levels of Metrnl stimulates energy expenditure and improves glucose tolerance and the expression of genes associated with beige fat thermogenesis and anti-inflammatory cytokines. Metrnl stimulates an eosinophil-dependent increase in IL-4 expression and promotes alternative activation of adipose tissue macrophages, which are required for the increased expression of the thermogenic and anti-inflammatory gene programs in fat. Importantly, blocking Metrnl actions in vivo significantly attenuates chronic cold-exposure-induced alternative macrophage activation and thermogenic gene responses. Thus, Metrnl links host-adaptive responses to the regulation of energy homeostasis and tissue inflammation and has therapeutic potential for metabolic and inflammatory diseases.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures
Comment in
-
Eosinophils in fat: pink is the new brown.Cell. 2014 Jun 5;157(6):1249-1250. doi: 10.1016/j.cell.2014.05.025. Cell. 2014. PMID: 24906141
-
Immunometabolism: a beige immune response.Nat Rev Immunol. 2014 Jul;14(7):433. doi: 10.1038/nri3706. Epub 2014 Jun 13. Nat Rev Immunol. 2014. PMID: 24925140 No abstract available.
-
Metabolism: Type 2 immunity at the origin of beige adipocytes.Nat Rev Endocrinol. 2014 Aug;10(8):443. doi: 10.1038/nrendo.2014.99. Epub 2014 Jun 24. Nat Rev Endocrinol. 2014. PMID: 24958309 No abstract available.
References
-
- Akimoto T, Pohnert SC, Li P, Zhang M, Gumbs C, Rosenberg PB, Williams RS, Yan Z. Exercise stimulates Pgc-1alpha transcription in skeletal muscle through activation of the p38 MAPK pathway. The Journal of biological chemistry. 2005;280:19587–19593. - PubMed
-
- Cederberg A, Gronning LM, Ahren B, Tasken K, Carlsson P, Enerback S. FOXC2 is a winged helix gene that counteracts obesity, hypertriglyceridemia, and diet-induced insulin resistance. Cell. 2001;106:563–573. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

