Hippo pathway activity influences liver cell fate
- PMID: 24906150
- PMCID: PMC4136468
- DOI: 10.1016/j.cell.2014.03.060
Hippo pathway activity influences liver cell fate
Abstract
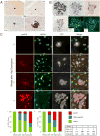
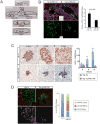
The Hippo-signaling pathway is an important regulator of cellular proliferation and organ size. However, little is known about the role of this cascade in the control of cell fate. Employing a combination of lineage tracing, clonal analysis, and organoid culture approaches, we demonstrate that Hippo pathway activity is essential for the maintenance of the differentiated hepatocyte state. Remarkably, acute inactivation of Hippo pathway signaling in vivo is sufficient to dedifferentiate, at very high efficiencies, adult hepatocytes into cells bearing progenitor characteristics. These hepatocyte-derived progenitor cells demonstrate self-renewal and engraftment capacity at the single-cell level. We also identify the NOTCH-signaling pathway as a functional important effector downstream of the Hippo transducer YAP. Our findings uncover a potent role for Hippo/YAP signaling in controlling liver cell fate and reveal an unprecedented level of phenotypic plasticity in mature hepatocytes, which has implications for the understanding and manipulation of liver regeneration.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures





Comment in
-
Prometheus and progenitors.Hepatology. 2015 Apr;61(4):1427-9. doi: 10.1002/hep.27676. Epub 2015 Feb 13. Hepatology. 2015. PMID: 25545250 Free PMC article. No abstract available.
References
-
- Camargo FD, Gokhale S, Johnnidis JB, Fu D, Bell GW, Jaenisch R, Brummelkamp TR. YAP1 increases organ size and expands undifferentiated progenitor cells. Current Biology. 2007;17:2054–2060. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

