The HP1 homolog rhino anchors a nuclear complex that suppresses piRNA precursor splicing
- PMID: 24906152
- PMCID: PMC4167631
- DOI: 10.1016/j.cell.2014.04.030
The HP1 homolog rhino anchors a nuclear complex that suppresses piRNA precursor splicing
Abstract
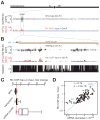
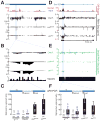
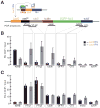
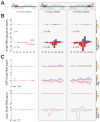
piRNAs guide an adaptive genome defense system that silences transposons during germline development. The Drosophila HP1 homolog Rhino is required for germline piRNA production. We show that Rhino binds specifically to the heterochromatic clusters that produce piRNA precursors, and that binding directly correlates with piRNA production. Rhino colocalizes to germline nuclear foci with Rai1/DXO-related protein Cuff and the DEAD box protein UAP56, which are also required for germline piRNA production. RNA sequencing indicates that most cluster transcripts are not spliced and that rhino, cuff, and uap56 mutations increase expression of spliced cluster transcripts over 100-fold. LacI::Rhino fusion protein binding suppresses splicing of a reporter transgene and is sufficient to trigger piRNA production from a trans combination of sense and antisense reporters. We therefore propose that Rhino anchors a nuclear complex that suppresses cluster transcript splicing and speculate that stalled splicing differentiates piRNA precursors from mRNAs.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures



Comment in
-
Getting a grip on piRNA cluster transcription.Cell. 2014 Jun 5;157(6):1253-1254. doi: 10.1016/j.cell.2014.05.022. Cell. 2014. PMID: 24906143 Free PMC article.
References
-
- Aravin A, Gaidatzis D, Pfeffer S, Lagos-Quintana M, Landgraf P, Iovino N, Morris P, Brownstein MJ, Kuramochi-Miyagawa S, Nakano T, Chien M, Russo JJ, Ju J, Sheridan R, Sander C, Zavolan M, Tuschl T. A novel class of small RNAs bind to MILI protein in mouse testes. Nature. 2006;442:203–207. - PubMed
-
- Bennetzen JL. Transposable element contributions to plant gene and genome evolution. Plant Mol Biol. 2000;42:251–269. - PubMed
-
- Brennecke J, Aravin AA, Stark A, Dus M, Kellis M, Sachidanandam R, Hannon GJ. Discrete small RNA-generating loci as master regulators of transposon activity in Drosophila. Cell. 2007;128:1089–1103. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

