Bromine is an essential trace element for assembly of collagen IV scaffolds in tissue development and architecture
- PMID: 24906154
- PMCID: PMC4144415
- DOI: 10.1016/j.cell.2014.05.009
Bromine is an essential trace element for assembly of collagen IV scaffolds in tissue development and architecture
Abstract
Bromine is ubiquitously present in animals as ionic bromide (Br(-)) yet has no known essential function. Herein, we demonstrate that Br(-) is a required cofactor for peroxidasin-catalyzed formation of sulfilimine crosslinks, a posttranslational modification essential for tissue development and architecture found within the collagen IV scaffold of basement membranes (BMs). Bromide, converted to hypobromous acid, forms a bromosulfonium-ion intermediate that energetically selects for sulfilimine formation. Dietary Br deficiency is lethal in Drosophila, whereas Br replenishment restores viability, demonstrating its physiologic requirement. Importantly, Br-deficient flies phenocopy the developmental and BM defects observed in peroxidasin mutants and indicate a functional connection between Br(-), collagen IV, and peroxidasin. We establish that Br(-) is required for sulfilimine formation within collagen IV, an event critical for BM assembly and tissue development. Thus, bromine is an essential trace element for all animals, and its deficiency may be relevant to BM alterations observed in nutritional and smoking-related disease. PAPERFLICK:
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures

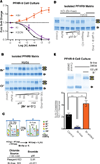




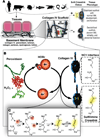
References
-
- Anke M, Regius Á, Groppel B, Arnhold W. Essentiality of the trace element bromine. Acta Agronomica Hung. 1990;39:297–303.
-
- Armesto XL, Canle LM, Fernandez MI, Garca MV, Santaballa JA. First Steps in the Oxidation of Sulfur-Containing Amino Acids by Hypohalogenation: Very Fast Generation of Intermediate Sulfenyl Halides and Halosulfonium Cations. Tetrahedron. 2000;56:1103–1109.
-
- Asmussen I. Fetal cardiovascular system as influenced by maternal smoking. Clin Cardiol. 1979;2:246–256. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

