A "push and slide" mechanism allows sequence-insensitive translocation of secretory proteins by the SecA ATPase
- PMID: 24906156
- PMCID: PMC4104599
- DOI: 10.1016/j.cell.2014.03.063
A "push and slide" mechanism allows sequence-insensitive translocation of secretory proteins by the SecA ATPase
Abstract
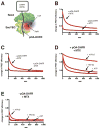
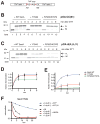
In bacteria, most secretory proteins are translocated across the plasma membrane by the interplay of the SecA ATPase and the SecY channel. How SecA moves a broad range of polypeptide substrates is only poorly understood. Here we show that SecA moves polypeptides through the SecY channel by a "push and slide" mechanism. In its ATP-bound state, SecA interacts through a two-helix finger with a subset of amino acids in a substrate, pushing them into the channel. A polypeptide can also passively slide back and forth when SecA is in the predominant ADP-bound state or when SecA encounters a poorly interacting amino acid in its ATP-bound state. SecA performs multiple rounds of ATP hydrolysis before dissociating from SecY. The proposed push and slide mechanism is supported by a mathematical model and explains how SecA allows translocation of a wide range of polypeptides. This mechanism may also apply to hexameric polypeptide-translocating ATPases.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures





References
-
- DeLaBarre B, Christianson JC, Kopito RR, Brunger AT. Central Pore Residues Mediate the p97/VCP Activity Required for ERAD. Mol Cell. 2006;22:451–462. - PubMed
-
- Economou A, Pogliano JA, Beckwith J, Oliver DB, Wickner W. SecA membrane cycling at SecYEG is driven by distinct ATP binding and hydrolysis events and is regulated by SecD and SecF. Cell. 1995;83:1171–1181. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

