Cell cycle regulation during viral infection
- PMID: 24906315
- PMCID: PMC7122065
- DOI: 10.1007/978-1-4939-0888-2_10
Cell cycle regulation during viral infection
Abstract
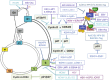
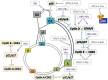
To replicate their genomes in cells and generate new progeny, viruses typically require factors provided by the cells that they have infected. Subversion of the cellular machinery that controls replication of the infected host cell is a common activity of many viruses. Viruses employ different strategies to deregulate cell cycle checkpoint controls and modulate cell proliferation pathways. A number of DNA and RNA viruses encode proteins that target critical cell cycle regulators to achieve cellular conditions that are beneficial for viral replication. Many DNA viruses induce quiescent cells to enter the cell cycle; this is thought to increase pools of deoxynucleotides and thus, facilitate viral replication. In contrast, some viruses can arrest cells in a particular phase of the cell cycle that is favorable for replication of the specific virus. Cell cycle arrest may inhibit early cell death of infected cells, allow the cells to evade immune defenses, or help promote virus assembly. Although beneficial for the viral life cycle, virus-mediated alterations in normal cell cycle control mechanisms could have detrimental effects on cellular physiology and may ultimately contribute to pathologies associated with the viral infection, including cell transformation and cancer progression and maintenance. In this chapter, we summarize various strategies employed by DNA and RNA viruses to modulate the replication cycle of the virus-infected cell. When known, we describe how these virus-associated effects influence replication of the virus and contribute to diseases associated with infection by that specific virus.
Figures



References
-
- Nascimento R, Costa H, Parkhouse RM. Virus manipulation of cell cycle. Protoplasma. 2012;249(3):519–528. - PubMed
-
- Condit RC (2013) Principles of virology. In: Knipe DM, Howley PM (eds) Fields virology, vol 1, 6th edn. Lippincot Williams and Wilkins, Philadelphia, PA, pp 21–51
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

