The roles of cohesins in mitosis, meiosis, and human health and disease
- PMID: 24906316
- PMCID: PMC4495907
- DOI: 10.1007/978-1-4939-0888-2_11
The roles of cohesins in mitosis, meiosis, and human health and disease
Abstract
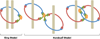
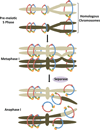
Mitosis and meiosis are essential processes that occur during development. Throughout these processes, cohesion is required to keep the sister chromatids together until their separation at anaphase. Cohesion is created by multiprotein subunit complexes called cohesins. Although the subunits differ slightly in mitosis and meiosis, the canonical cohesin complex is composed of four subunits that are quite diverse. The cohesin complexes are also important for DNA repair, gene expression, development, and genome integrity. Here we provide an overview of the roles of cohesins during these different events as well as their roles in human health and disease, including the cohesinopathies. Although the exact roles and mechanisms of these proteins are still being elucidated, this review serves as a guide for the current knowledge of cohesins.
Figures



References
-
- Tanaka T, Fuchs J, Loidl J, Nasmyth K. Cohesin ensures bipolar attachment of microtubules to sister centromeres and resists their precocious separation. Nat Cell Biol. 2000;2:492–499. - PubMed
-
- Uhlmann F, Lottspeich F, Nasmyth K. Sister-chromatid separation at anaphase onset is promoted by cleavage of the cohesin subunit Scc1. Nature. 1999;400:37–42. - PubMed
-
- Michaelis C, Ciosk R, Nasmyth K. Cohesins: chromosomal proteins that prevents premature separation of sister chromatids. Cell. 1997;91:35–45. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

