Transcranial cerebellar direct current stimulation and transcutaneous spinal cord direct current stimulation as innovative tools for neuroscientists
- PMID: 24907311
- PMCID: PMC4229333
- DOI: 10.1113/jphysiol.2013.270280
Transcranial cerebellar direct current stimulation and transcutaneous spinal cord direct current stimulation as innovative tools for neuroscientists
Abstract
Two neuromodulatory techniques based on applying direct current (DC) non-invasively through the skin, transcranial cerebellar direct current stimulation (tDCS) and transcutaneous spinal DCS, can induce prolonged functional changes consistent with a direct influence on the human cerebellum and spinal cord. In this article we review the major experimental works on cerebellar tDCS and on spinal tDCS, and their preliminary clinical applications. Cerebellar tDCS modulates cerebellar motor cortical inhibition, gait adaptation, motor behaviour, and cognition (learning, language, memory, attention). Spinal tDCS influences the ascending and descending spinal pathways, and spinal reflex excitability. In the anaesthetised mouse, DC stimulation applied under the skin along the entire spinal cord may affect GABAergic and glutamatergic systems. Preliminary clinical studies in patients with cerebellar disorders, and in animals and patients with spinal cord injuries, have reported beneficial effects. Overall the available data show that cerebellar tDCS and spinal tDCS are two novel approaches for inducing prolonged functional changes and neuroplasticity in the human cerebellum and spinal cord, and both are new tools for experimental and clinical neuroscientists.
© 2014 The Authors. The Journal of Physiology © 2014 The Physiological Society.
Figures

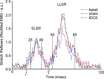

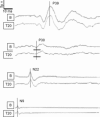


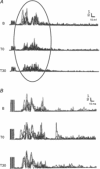
References
-
- Ahmed Z. Trans-spinal direct current stimulation modulates motor cortex-induced muscle contraction in mice. J Appl Physiol (1985) 2011;110:1414–1424. - PubMed
-
- Ahmed Z. Wieraszko A. Trans-spinal direct current enhances corticospinal output and stimulation-evoked release of glutamate analog, d-2,3-3H-aspartic acid. J Appl Physiol (1985) 2012;112:1576–1592. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

