Vertebrate decomposition is accelerated by soil microbes
- PMID: 24907317
- PMCID: PMC4135757
- DOI: 10.1128/AEM.00957-14
Vertebrate decomposition is accelerated by soil microbes
Abstract
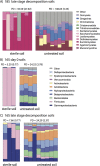
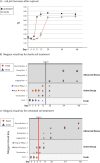
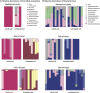
Carrion decomposition is an ecologically important natural phenomenon influenced by a complex set of factors, including temperature, moisture, and the activity of microorganisms, invertebrates, and scavengers. The role of soil microbes as decomposers in this process is essential but not well understood and represents a knowledge gap in carrion ecology. To better define the role and sources of microbes in carrion decomposition, lab-reared mice were decomposed on either (i) soil with an intact microbial community or (ii) soil that was sterilized. We characterized the microbial community (16S rRNA gene for bacteria and archaea, and the 18S rRNA gene for fungi and microbial eukaryotes) for three body sites along with the underlying soil (i.e., gravesoils) at time intervals coinciding with visible changes in carrion morphology. Our results indicate that mice placed on soil with intact microbial communities reach advanced stages of decomposition 2 to 3 times faster than those placed on sterile soil. Microbial communities associated with skin and gravesoils of carrion in stages of active and advanced decay were significantly different between soil types (sterile versus untreated), suggesting that substrates on which carrion decompose may partially determine the microbial decomposer community. However, the source of the decomposer community (soil- versus carcass-associated microbes) was not clear in our data set, suggesting that greater sequencing depth needs to be employed to identify the origin of the decomposer communities in carrion decomposition. Overall, our data show that soil microbial communities have a significant impact on the rate at which carrion decomposes and have important implications for understanding carrion ecology.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures



References
-
- Payne JA. 1965. A summer carrion study of the baby pig Sus scrofa Linneaeus. Ecology 46:592–602. 10.2307/1934999 - DOI
-
- Dilly O, Bartsch S, Rosenbrock P, Buscot F, Munch JC. 2001. Shifts in physiological capabilities of the microbiota during the decomposition of leaf litter in a black alder (Alnus glutinosa (Gaertn.) L.) forest. Soil Biol. Biochem. 33:921–930. 10.1016/S0038-0717(00)00239-X - DOI
-
- Sinsabaugh RL, Lauber CL, Weintraub MN, Ahmed B, Allison SD, Crenshaw C, Contosta AR, Cusack D, Frey S, Gallo ME, Gartner TB, Hobbie SE, Holland K, Keeler BL, Powers JS, Stursova M, Takacs-Vesbach C, Waldrop MP, Wallenstein MD, Zak DR, Zeglin LH. 2008. Stoichiometry of soil enzyme activity at global scale. Ecol. Lett. 11:1252–1264. 10.1111/j.1461-0248.2008.01245.x - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

