The cell-cell junctions of mammalian testes: I. The adhering junctions of the seminiferous epithelium represent special differentiation structures
- PMID: 24907851
- PMCID: PMC4148596
- DOI: 10.1007/s00441-014-1906-9
The cell-cell junctions of mammalian testes: I. The adhering junctions of the seminiferous epithelium represent special differentiation structures
Abstract
The seminiferous tubules and the excurrent ducts of the mammalian testis are physiologically separated from the mesenchymal tissues and the blood and lymph system by a special structural barrier to paracellular translocations of molecules and particles: the "blood-testis barrier", formed by junctions connecting Sertoli cells with each other and with spermatogonial cells. In combined biochemical as well as light and electron microscopical studies we systematically determine the molecules located in the adhering junctions of adult mammalian (human, bovine, porcine, murine, i.e., rat and mouse) testis. We show that the seminiferous epithelium does not contain desmosomes, or "desmosome-like" junctions, nor any of the desmosome-specific marker molecules and that the adhering junctions of tubules and ductules are fundamentally different. While the ductules contain classical epithelial cell layers with E-cadherin-based adherens junctions (AJs) and typical desmosomes, the Sertoli cells of the tubules lack desmosomes and "desmosome-like" junctions but are connected by morphologically different forms of AJs. These junctions are based on N-cadherin anchored in cytoplasmic plaques, which in some subforms appear thick and dense but in other subforms contain only scarce and loosely arranged plaque structures formed by α- and β-catenin, proteins p120, p0071 and plakoglobin, together with a member of the striatin family and also, in rodents, the proteins ZO-1 and myozap. These N-cadherin-based AJs also include two novel types of junctions: the "areae adhaerentes", i.e., variously-sized, often very large cell-cell contacts and small sieve-plate-like AJs perforated by cytoplasm-to-cytoplasm channels of 5-7 nm internal diameter ("cribelliform junctions"). We emphasize the unique character of this epithelium that totally lacks major epithelial marker molecules and structures such as keratin filaments and desmosomal elements as well as EpCAM- and PERP-containing junctions. We also discuss the nature, development and possible functions of these junctions.
Figures



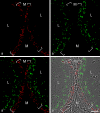

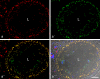






Comment in
-
The cell-cell junctions of mammalian testes-a summary.Cell Tissue Res. 2020 Jan;379(1):73-74. doi: 10.1007/s00441-019-03150-3. Cell Tissue Res. 2020. PMID: 31858240 No abstract available.
References
-
- Akat K, Bleck CKE, Lee Y-MA, Haselmann-Weiss U, Kartenbeck J. Characterization of a novel type of adherens junction in meningiomas and the derived cell line HBL-52. Cell Tissue Res. 2008;331:401–412. - PubMed
-
- Altorfer J, Fukuda T, Hedinger C. Desmosomes in human seminiferous epithelium. An electron microscopic study. Virchows Arch B. 1974;16:181–194. - PubMed
-
- Baccetti B, Bigliardi E, Talluri MV, Burrini AG. The Sertoli cell in lizards. J Ultrastruct Res. 1983;85:11–23. - PubMed
-
- Barth M, Schumacher H, Kuhn C, Akhyari P, Lichtenberg A, Franke WW. Cordial connections: molecular ensembles and structures of adhering junctions connecting interstitial cells of cardiac valves in situ and in cell culture. Cell Tissue Res. 2009;337:63–77. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

