The role of BMPs in endothelial cell function and dysfunction
- PMID: 24908616
- PMCID: PMC4149816
- DOI: 10.1016/j.tem.2014.05.003
The role of BMPs in endothelial cell function and dysfunction
Abstract
The bone morphogenetic protein (BMP) family of proteins has a multitude of roles throughout the body. In embryonic development, BMPs promote endothelial specification and subsequent venous differentiation. The BMP pathway also plays important roles in the adult vascular endothelium, promoting angiogenesis and mediating shear and oxidative stress. The canonical BMP pathway functions through the Smad transcription factors; however, other intracellular signaling cascades can be activated, and receptor complexes beyond the traditional type I and type II receptors add additional layers of regulation. Dysregulated BMP signaling has been linked to vascular diseases including pulmonary hypertension and atherosclerosis. This review addresses recent advances in the roles of BMP signaling in the endothelium and how BMPs affect endothelial dysfunction and human disease.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures
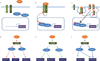
References
-
- Cai J, et al. BMP signaling in vascular diseases. FEBS Lett. 2012;586:1993–2002. - PubMed
-
- Bandyopadhyay A, et al. BMP signaling in development and diseases: a pharmacological perspective. Biochemical pharmacology. 2013;85:857–864. - PubMed
-
- Maddaluno L, et al. EndMT contributes to the onset and progression of cerebral cavernous malformations. Nature. 2013;498:492–496. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

