Loss of Tau results in defects in photoreceptor development and progressive neuronal degeneration in Drosophila
- PMID: 24909306
- PMCID: PMC4212004
- DOI: 10.1002/dneu.22199
Loss of Tau results in defects in photoreceptor development and progressive neuronal degeneration in Drosophila
Abstract
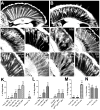
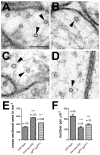
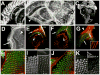
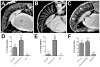
Accumulations of Tau, a microtubule-associated protein (MAP), into neurofibrillary tangles is a hallmark of Alzheimer's disease and other tauopathies. However, the mechanisms leading to this pathology are still unclear: the aggregates themselves could be toxic or the sequestration of Tau into tangles might prevent Tau from fulfilling its normal functions, thereby inducing a loss of function defect. Surprisingly, the consequences of losing normal Tau expression in vivo are still not well understood, in part due to the fact that Tau knockout mice show only subtle phenotypes, presumably due to the fact that mammals express several MAPs with partially overlapping functions. In contrast, flies express fewer MAP, with Tau being the only member of the Tau/MAP2/MAP4 family. Therefore, we used Drosophila to address the physiological consequences caused by the loss of Tau. Reducing the levels of fly Tau (dTau) ubiquitously resulted in developmental lethality, whereas deleting Tau specifically in neurons or the eye caused progressive neurodegeneration. Similarly, chromosomal mutations affecting dTau also caused progressive degeneration in both the eye and brain. Although photoreceptor cells initially developed normally in dTau knockdown animals, they subsequently degenerated during late pupal stages whereas weaker dTau alleles caused an age-dependent defect in rhabdomere structure. Expression of wild type human Tau partially rescued the neurodegenerative phenotype caused by the loss of endogenous dTau, suggesting that the functions of Tau proteins are functionally conserved from flies to humans.
Keywords: Drosophila; eye development; neurodegeneration; tau.
© 2014 Wiley Periodicals, Inc.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Drosophila Tau Negatively Regulates Translation and Olfactory Long-Term Memory, But Facilitates Footshock Habituation and Cytoskeletal Homeostasis.J Neurosci. 2019 Oct 16;39(42):8315-8329. doi: 10.1523/JNEUROSCI.0391-19.2019. Epub 2019 Sep 5. J Neurosci. 2019. PMID: 31488613 Free PMC article.
-
Deletion of endogenous Tau proteins is not detrimental in Drosophila.Sci Rep. 2016 Mar 15;6:23102. doi: 10.1038/srep23102. Sci Rep. 2016. PMID: 26976084 Free PMC article.
-
Loss of axonal mitochondria promotes tau-mediated neurodegeneration and Alzheimer's disease-related tau phosphorylation via PAR-1.PLoS Genet. 2012;8(8):e1002918. doi: 10.1371/journal.pgen.1002918. Epub 2012 Aug 30. PLoS Genet. 2012. PMID: 22952452 Free PMC article.
-
Cellular and molecular modifier pathways in tauopathies: the big picture from screening invertebrate models.J Neurochem. 2016 Apr;137(1):12-25. doi: 10.1111/jnc.13532. Epub 2016 Feb 11. J Neurochem. 2016. PMID: 26756400 Review.
-
Tau pathology in Alzheimer disease and other tauopathies.Biochim Biophys Acta. 2005 Jan 3;1739(2-3):198-210. doi: 10.1016/j.bbadis.2004.09.008. Biochim Biophys Acta. 2005. PMID: 15615638 Review.
Cited by
-
Drosophila Tau Negatively Regulates Translation and Olfactory Long-Term Memory, But Facilitates Footshock Habituation and Cytoskeletal Homeostasis.J Neurosci. 2019 Oct 16;39(42):8315-8329. doi: 10.1523/JNEUROSCI.0391-19.2019. Epub 2019 Sep 5. J Neurosci. 2019. PMID: 31488613 Free PMC article.
-
Human Tau Expression Does Not Induce Mouse Retina Neurodegeneration, Suggesting Differential Toxicity of Tau in Brain vs. Retinal Neurons.Front Mol Neurosci. 2018 Aug 24;11:293. doi: 10.3389/fnmol.2018.00293. eCollection 2018. Front Mol Neurosci. 2018. PMID: 30197586 Free PMC article.
-
Eosin whole-brain mount staining to analyze neurodegeneration in a fly model of Alzheimer's disease.STAR Protoc. 2022 May 25;3(2):101377. doi: 10.1016/j.xpro.2022.101377. eCollection 2022 Jun 17. STAR Protoc. 2022. PMID: 35634356 Free PMC article.
-
FTD-associated mutations in Tau result in a combination of dominant and recessive phenotypes.Neurobiol Dis. 2022 Aug;170:105770. doi: 10.1016/j.nbd.2022.105770. Epub 2022 May 16. Neurobiol Dis. 2022. PMID: 35588988 Free PMC article.
-
Visual impairment and progressive phthisis bulbi caused by recessive pathogenic variant in MARK3.Hum Mol Genet. 2018 Aug 1;27(15):2703-2711. doi: 10.1093/hmg/ddy180. Hum Mol Genet. 2018. PMID: 29771303 Free PMC article.
References
-
- Barten DM, Fanara P, Andorfer C, Hoque N, Wong PY, Husted KH, Cadelina GW, Decarr LB, Yang L, Liu V, Fessler C, Protassio J, Riff T, Turner H, Janus CG, Sankaranarayanan S, Polson C, Meredith JE, Gray G, Hanna A, Olson RE, Kim SH, Vite GD, Lee FY, Albright CF. Hyperdynamic microtubules, cognitive deficits, and pathology are improved in tau transgenic mice with low doses of the microtubule-stabilizing agent BMS-241027. J Neurosci. 2012;32:7137–7145. - PMC - PubMed
-
- Bettencourt da Cruz A, Schwarzel M, Schulze S, Niyyati M, Heisenberg M, Kretzschmar D. Disruption of the MAP1B-related protein FUTSCH leads to changes in the neuronal cytoskeleton, axonal transport defects, and progressive neurodegeneration in Drosophila. Mol Biol Cell. 2005;16:2433–2442. - PMC - PubMed
-
- Cash AD, Aliev G, Siedlak SL, Nunomura A, Fujioka H, Zhu X, Raina AK, Vinters HV, Tabaton M, Johnson AB, Paula-Barbosa M, Avila J, Jones PK, Castellani RJ, Smith MA, Perry G. Microtubule reduction in Alzheimer’s disease and aging is independent of tau filament formation. Am J Pathol. 2003;162:1623–1627. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

