Restricted VH/VL usage and limited mutations in gluten-specific IgA of coeliac disease lesion plasma cells
- PMID: 24909383
- PMCID: PMC4059925
- DOI: 10.1038/ncomms5041
Restricted VH/VL usage and limited mutations in gluten-specific IgA of coeliac disease lesion plasma cells
Abstract
Coeliac disease (CD), an enteropathy caused by cereal gluten ingestion, is characterized by CD4(+) T cells recognizing deamidated gluten and by antibodies reactive to gluten or the self-antigen transglutaminase 2 (TG2). TG2-specific immunoglobulin A (IgA) of plasma cells (PCs) from CD lesions have limited somatic hypermutation (SHM). Here we report that gluten-specific IgA of lesion-resident PCs share this feature. Monoclonal antibodies were expression cloned from single PCs of patients either isolated from cultures with reactivity to complex deamidated gluten antigen or by sorting with gluten peptide tetramers. Typically, the antibodies bind gluten peptides related to T-cell epitopes and many have higher reactivity to deamidated peptides. There is restricted VH and VL combination and usage among the antibodies. Limited SHM suggests that a common factor governs the mutation level in PCs producing TG2- and gluten-specific IgA. The antibodies have potential use for diagnosis of CD and for detection of gluten.
Conflict of interest statement
Ø.S., L.M.S., C.J.H.D. and P.W. have submitted a patent application covering gliadin-specific monoclonal antibodies. The remaining authors declare no competing financial interests.
Figures
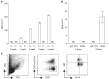





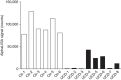
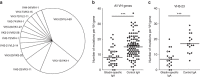
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

