Direct acute tubular damage contributes to Shigatoxin-mediated kidney failure
- PMID: 24909663
- PMCID: PMC4282478
- DOI: 10.1002/path.4388
Direct acute tubular damage contributes to Shigatoxin-mediated kidney failure
Abstract
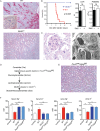
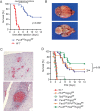
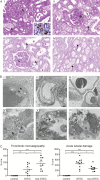
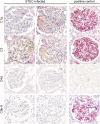
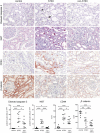
The pathogenesis and therapy of Shigatoxin 2 (Stx2)-mediated kidney failure remain controversial. Our aim was to test whether, during an infection with Stx2-producing E. coli (STEC), Stx2 exerts direct effects on renal tubular epithelium and thereby possibly contributes to acute renal failure. Mice represent a suitable model because they, like humans, express the Stx2-receptor Gb3 in the tubular epithelium but, in contrast to humans, not in glomerular endothelia, and are thus free of glomerular thrombotic microangiopathy (TMA). In wild-type mice, Stx2 caused acute tubular dysfunction with consequent electrolyte disturbance, which was most likely the cause of death. Tubule-specific depletion of Gb3 protected the mice from acute renal failure. In vitro, Stx2 induced secretion of proinflammatory cytokines and apoptosis in human tubular epithelial cells, thus implicating a direct effect of Stx2 on the tubular epithelium. To correlate these results to human disease, kidney biopsies and outcome were analysed in patients with Stx2-associated kidney failure (n = 11, aged 22-44 years). The majority of kidney biopsies showed different stages of an ongoing TMA; however, no glomerular complement activation could be demonstrated. All biopsies, including those without TMA, showed severe acute tubular damage. Due to these findings, patients were treated with supportive therapy without complement-inhibiting antibodies (eculizumab) or immunoadsorption. Despite the severity of the initial disease [creatinine 6.34 (1.31-17.60) mg/dl, lactate dehydrogenase 1944 (753-2792) U/l, platelets 33 (19-124)/nl and haemoglobin 6.2 (5.2-7.8) g/dl; median (range)], all patients were discharged after 33 (range 19-43) days with no neurological symptoms and no dialysis requirement [creatinine 1.39 (range 0.84-2.86) mg/dl]. The creatinine decreased further to 0.90 (range 0.66-1.27) mg/dl after 24 months. Based on these data, one may surmise that acute tubular damage represents a separate pathophysiological mechanism, importantly contributing to Stx2-mediated acute kidney failure. Specifically in young adults, an excellent outcome can be achieved by supportive therapy only.
Keywords: Shigatoxin; Shigatoxin-producing Escherichia coli (STEC); acute renal failure; acute tubular damage; electron microscopy; globoside (Gb3, CD77); thrombotic microangiopathy.
© 2014 The Authors. The Journal of Pathology published by John Wiley & Sons Ltd on behalf of Pathological Society of Great Britain and Ireland.
Figures

References
-
- Riley LW, Remis RS, Helgerson SD, et al. Hemorrhagic colitis associated with a rare Escherichia coli serotype. N Engl J Med. 1983;308:681–685. - PubMed
-
- Karmali MA, Petric M, Lim C, et al. The association between idiopathic hemolytic uremic syndrome and infection by verotoxin-producing Escherichia coli. J Infect Dis. 1985;151:775–782. - PubMed
-
- MMWR. Update: multistate outbreak of Escherichia coli O157:H7 infections from hamburgers – western United States, 1992–1993. MMWR Morb Mortal Wkly Rep. 42:258–263. - PubMed
-
- Michino H, Araki K, Minami S, et al. Massive outbreak of Escherichia coli O157:H7 infection in schoolchildren in Sakai City, Japan, associated with consumption of white radish sprouts. Am J Epidemiol. 1999;150:787–796. - PubMed
-
- Thorpe CM. Shiga toxin-producing Escherichia coli infection. Clin Infect Dis. 2004;38:1298–1303. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

