B cells regulate macrophage phenotype and response to chemotherapy in squamous carcinomas
- PMID: 24909985
- PMCID: PMC4063283
- DOI: 10.1016/j.ccr.2014.04.026
B cells regulate macrophage phenotype and response to chemotherapy in squamous carcinomas
Abstract
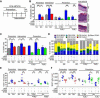
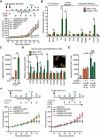
B cells foster squamous cell carcinoma (SCC) development through deposition of immunoglobulin-containing immune complexes in premalignant tissue and Fcγ receptor-dependent activation of myeloid cells. Because human SCCs of the vulva and head and neck exhibited hallmarks of B cell infiltration, we examined B cell-deficient mice and found reduced support for SCC growth. Although ineffective as a single agent, treatment of mice bearing preexisting SCCs with B cell-depleting αCD20 monoclonal antibodies improved response to platinum- and Taxol-based chemotherapy. Improved chemoresponsiveness was dependent on altered chemokine expression by macrophages that promoted tumor infiltration of activated CD8(+) lymphocytes via CCR5-dependent mechanisms. These data reveal that B cells, and the downstream myeloid-based pathways they regulate, represent tractable targets for anticancer therapy in select tumors.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures




References
-
- Albers A, Abe K, Hunt J, Wang J, Lopez-Albaitero A, Schaefer C, Gooding W, Whiteside TL, Ferrone S, DeLeo A, Ferris RL. Antitumor activity of human papillomavirus type 16 E7-specific T cells against virally infected squamous cell carcinoma of the head and neck. Cancer Res. 2005;65:11146–11155. - PubMed
-
- Arbeit JM, Olson DC, Hanahan D. Upregulation of fibroblast growth factors and their receptors during multi-stage epidermal carcinogenesis in K14-HPV16 transgenic mice. Oncogene. 1996;13:1847–1857. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
