Determination of kinetics and the crystal structure of a novel type 2 isopentenyl diphosphate: dimethylallyl diphosphate isomerase from Streptococcus pneumoniae
- PMID: 24910111
- PMCID: PMC4215930
- DOI: 10.1002/cbic.201402046
Determination of kinetics and the crystal structure of a novel type 2 isopentenyl diphosphate: dimethylallyl diphosphate isomerase from Streptococcus pneumoniae
Abstract
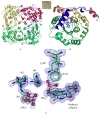
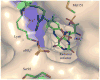
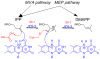
Isopentenyl diphosphate isomerase (IDI) is a key enzyme in the isoprenoid biosynthetic pathway and is required for all organisms that synthesize isoprenoid metabolites from mevalonate. Type 1 IDI (IDI-1) is a metalloprotein that is found in eukaryotes, whereas the type 2 isoform (IDI-2) is a flavoenzyme found in bacteria that is completely absent from human. IDI-2 from the pathogenic bacterium Streptococcus pneumoniae was recombinantly expressed in Escherichia coli. Steady-state kinetic studies of the enzyme indicated that FMNH2 (KM =0.3 μM) bound before isopentenyl diphosphate (KM =40 μM) in an ordered binding mechanism. An X-ray crystal structure at 1.4 Å resolution was obtained for the holoenzyme in the closed conformation with a reduced flavin cofactor and two sulfate ions in the active site. These results helped to further approach the enzymatic mechanism of IDI-2 and, thus, open new possibilities for the rational design of antibacterial compounds against sequence-similar and structure-related pathogens such as Enterococcus faecalis or Staphylococcus aureus.
Keywords: X-ray structures; bisubstrates; flavoproteins; isoprenoids; steady-state kinetics.
© 2014 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

