Aging and longevity in the simplest animals and the quest for immortality
- PMID: 24910306
- PMCID: PMC4133289
- DOI: 10.1016/j.arr.2014.05.003
Aging and longevity in the simplest animals and the quest for immortality
Abstract
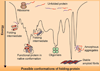
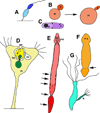
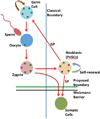
Here we review the examples of great longevity and potential immortality in the earliest animal types and contrast and compare these to humans and other higher animals. We start by discussing aging in single-celled organisms such as yeast and ciliates, and the idea of the immortal cell clone. Then we describe how these cell clones could become organized into colonies of different cell types that lead to multicellular animal life. We survey aging and longevity in all of the basal metazoan groups including ctenophores (comb jellies), sponges, placozoans, cnidarians (hydras, jellyfish, corals and sea anemones) and myxozoans. Then we move to the simplest bilaterian animals (with a head, three body cell layers, and bilateral symmetry), the two phyla of flatworms. A key determinant of longevity and immortality in most of these simple animals is the large numbers of pluripotent stem cells that underlie the remarkable abilities of these animals to regenerate and rejuvenate themselves. Finally, we discuss briefly the evolution of the higher bilaterians and how longevity was reduced and immortality lost due to attainment of greater body complexity and cell cycle strategies that protect these complex organisms from developing tumors. We also briefly consider how the evolution of multiple aging-related mechanisms/pathways hinders our ability to understand and modify the aging process in higher organisms.
Keywords: Bilateria; Cnidaria; Hydra; Metazoa; Neoblast; Planaria.
Published by Elsevier B.V.
Figures



References
-
- Ackermann M, Stearns SC, Jenal U. Senescence in a bacterium with asymmetric division. Science. 2003;300:1920. - PubMed
-
- Aitlhadj L, Sturzenbaum SR. Caenorhabditis elegans in regenerative medicine: a simple model for a complex discipline. Drug discovery today. 2014 - PubMed
-
- Alié A, Leclere L, Jager M, Dayraud C, Chang P, Le Guyader H, Queinnec E, Manuel M. Somatic stem cells express Piwi and Vasa genes in an adult ctenophore: ancient association of “germline genes” with stemness. Developmental biology. 2011;350:183–197. - PubMed
-
- Arumugam TV, Gleichmann M, Tang SC, Mattson MP. Hormesis/preconditioning mechanisms, the nervous system and aging. Ageing research reviews. 2006;5:165–178. - PubMed
-
- Ashworth JH, Annandale N. Observations on some aged specimens of Sagartia troglodytes and on the duration of life in Coelenterates. Proc Roy Soc Edin. 1904;25:295–308.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

