The impact of Nucleofection® on the activation state of primary human CD4 T cells
- PMID: 24910411
- PMCID: PMC4120863
- DOI: 10.1016/j.jim.2014.05.014
The impact of Nucleofection® on the activation state of primary human CD4 T cells
Abstract
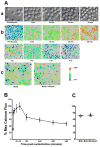
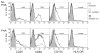
Gene transfer into primary human CD4 T lymphocytes is a critical tool in studying the mechanism of T cell-dependent immune responses and human immunodeficiency virus-1 (HIV-1) infection. Nucleofection® is an electroporation technique that allows efficient gene transfer into primary human CD4 T cells that are notoriously resistant to traditional electroporation. Despite its popularity in immunological research, careful characterization of its impact on the physiology of CD4 T cells has not been documented. Herein, using freshly-isolated primary human CD4 T cells, we examine the effects of Nucleofection® on CD4 T cell morphology, intracellular calcium levels, cell surface activation markers, and transcriptional activity. We find that immediately after Nucleofection®, CD4 T cells undergo dramatic morphological changes characterized by wrinkled and dilated plasma membranes before recovering 1h later. The intracellular calcium level also increases after Nucleofection®, peaking after 1h before recovering 8h post transfection. Moreover, Nucleofection® leads to increased expression of T cell activation markers, CD154 and CD69, for more than 24h, and enhances the activation effects of phytohemagglutinin (PHA) stimulation. In addition, transcriptional activity is increased in the first 24h after Nucleofection®, even in the absence of exogenous stimuli. Therefore, Nucleofection® significantly alters the activation state of primary human CD4 T cells. The effect of transferred gene products on CD4 T cell function by Nucleofection® should be assessed after sufficient resting time post transfection or analyzed in light of the activation caveats mentioned above.
Keywords: Calcium; Gene transfer; HIV-1; Nucleofection®; Plasmid; T cell activation.
Copyright © 2014 Elsevier B.V. All rights reserved.
Figures


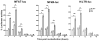
References
-
- Behre G, Smith LT, Tenen DG. Use of a promoterless Renilla luciferase vector as an internal control plasmid for transient co-transfection assays of Ras-mediated transcription activation. Biotechniques. 1999;26:24–6. 28. - PubMed
-
- Chiu YL, Soros VB, Kreisberg JF, Stopak K, Yonemoto W, Greene WC. Cellular APOBEC3G restricts HIV-1 infection in resting CD4+ T cells. Nature. 2005;435:108–14. - PubMed
-
- Crabtree GR, Olson EN. NFAT signaling: choreographing the social lives of cells. Cell. 2002;109(Suppl):S67–79. - PubMed
-
- Cron RQ. CD154 transcriptional regulation in primary human CD4 T cells. Immunol Res. 2003;27:185–202. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

