The Center for HIV/AIDS Vaccine Immunology (CHAVI) multi-site quality assurance program for cryopreserved human peripheral blood mononuclear cells
- PMID: 24910414
- PMCID: PMC4138272
- DOI: 10.1016/j.jim.2014.05.013
The Center for HIV/AIDS Vaccine Immunology (CHAVI) multi-site quality assurance program for cryopreserved human peripheral blood mononuclear cells
Abstract
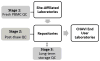
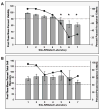
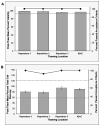
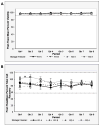
The Center for HIV/AIDS Vaccine Immunology (CHAVI) consortium was established to determine the host and virus factors associated with HIV transmission, infection and containment of virus replication, with the goal of advancing the development of an HIV protective vaccine. Studies to meet this goal required the use of cryopreserved Peripheral Blood Mononuclear Cell (PBMC) specimens, and therefore it was imperative that a quality assurance (QA) oversight program be developed to monitor PBMC samples obtained from study participants at multiple international sites. Nine site-affiliated laboratories in Africa and the USA collected and processed PBMCs, and cryopreserved PBMC were shipped to CHAVI repositories in Africa and the USA for long-term storage. A three-stage program was designed, based on Good Clinical Laboratory Practices (GCLP), to monitor PBMC integrity at each step of this process. The first stage evaluated the integrity of fresh PBMCs for initial viability, overall yield, and processing time at the site-affiliated laboratories (Stage 1); for the second stage, the repositories determined post-thaw viability and cell recovery of cryopreserved PBMC, received from the site-affiliated laboratories (Stage 2); the third stage assessed the long-term specimen storage at each repository (Stage 3). Overall, the CHAVI PBMC QA oversight program results highlight the relative importance of each of these stages to the ultimate goal of preserving specimen integrity from peripheral blood collection to long-term repository storage.
Keywords: Biorepository; Cryopreservation; HIV; Human clinical trials; PBMC; Vaccine.
Copyright © 2014 Elsevier B.V. All rights reserved.
Figures
References
-
- Afonso G, Scotto M, Renand A, Arvastsson J, Vassilieff D, Cilio CM, Mallone R. Critical parameters in blood processing for T-cell assays: validation on ELISpot and tetramer platforms. J Immunol Methods. 2010;359:28–36. - PubMed
-
- Cox JH, Ferrari G, Kalams SA, Lopaczynski W, Oden N, D’Souza MP E.C.S Group. Results of an ELISPOT proficiency panel conducted in 11 laboratories participating in international Human Immunodeficiency Virus Type 1 vaccine trials. AIDS Res Hum Retrovir. 2005;21:68–81. - PubMed
-
- Disis ML, dela Rosa C, Goodell V, Kuan LY, Chang JCC, Kuus-Reichel K, Clay TM, Lyerly HK, Bhatia S, Ghanekar SA, Maino VC, Maecker HT. Maximizing the retention of antigen specific lymphocyte function after cryopreservation. J Immunol Methods. 2006;308:13–18. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

