Age-related dysfunction in mechanotransduction impairs differentiation of human mammary epithelial progenitors
- PMID: 24910432
- PMCID: PMC4122253
- DOI: 10.1016/j.celrep.2014.05.021
Age-related dysfunction in mechanotransduction impairs differentiation of human mammary epithelial progenitors
Abstract
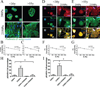
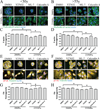
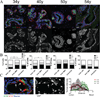
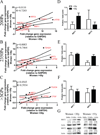
Dysfunctional progenitor and luminal cells with acquired basal cell properties accumulate during human mammary epithelial aging for reasons not understood. Multipotent progenitors from women aged <30 years were exposed to a physiologically relevant range of matrix elastic modulus (stiffness). Increased stiffness causes a differentiation bias towards myoepithelial cells while reducing production of luminal cells and progenitor maintenance. Lineage representation in progenitors from women >55 years is unaffected by physiological stiffness changes. Efficient activation of Hippo pathway transducers YAP and TAZ is required for the modulus-dependent myoepithelial/basal bias in younger progenitors. In older progenitors, YAP and TAZ are activated only when stressed with extraphysiologically stiff matrices, which bias differentiation towards luminal-like phenotypes. In vivo YAP is primarily active in myoepithelia of younger breasts, but localization and activity increases in luminal cells with age. Thus, aging phenotypes of mammary epithelia may arise partly because alterations in Hippo pathway activation impair microenvironment-directed differentiation and lineage specificity.
Copyright © 2014 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Bershadsky AD, Balaban NQ, Geiger B. Adhesion-dependent cell mechanosensitivity. Annual review of cell and developmental biology. 2003;19:677–695. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

