MtDNA segregation in heteroplasmic tissues is common in vivo and modulated by haplotype differences and developmental stage
- PMID: 24910436
- PMCID: PMC4570183
- DOI: 10.1016/j.celrep.2014.05.020
MtDNA segregation in heteroplasmic tissues is common in vivo and modulated by haplotype differences and developmental stage
Abstract
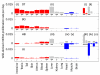
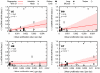
The dynamics by which mitochondrial DNA (mtDNA) evolves within organisms are still poorly understood, despite the fact that inheritance and proliferation of mutated mtDNA cause fatal and incurable diseases. When two mtDNA haplotypes are present in a cell, it is usually assumed that segregation (the proliferation of one haplotype over another) is negligible. We challenge this assumption by showing that segregation depends on the genetic distance between haplotypes. We provide evidence by creating four mouse models containing mtDNA haplotype pairs of varying diversity. We find tissue-specific segregation in all models over a wide range of tissues. Key findings are segregation in postmitotic tissues (important for disease models) and segregation covering all developmental stages from prenatal to old age. We identify four dynamic regimes of mtDNA segregation. Our findings suggest potential complications for therapies in human populations: we propose "haplotype matching" as an approach to avoid these issues.
Copyright © 2014 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Acton BM, Lai I, Shang X, Jurisicova A, Casper RF. Neutral mitochondrial heteroplasmy alters physiological function in mice. Biol Reprod. 2007;77:569–576. - PubMed
-
- Battersby BJ, Loredo-Osti JC, Shoubridge EA. Nuclear genetic control of mitochondrial DNA segregation. Nat Genet. 2003;33:183–186. - PubMed
-
- Battersby BJ, Shoubridge EA. Selection of a mtDNA sequence variant in hepatocytes of heteroplasmic mice is not due to differences in respiratory chain function or efficiency of replication. Hum Mol Genet. 2001;10:2469–2479. - PubMed
-
- Battersby BJ, Shoubridge EA. Reactive oxygen species and the segregation of mtDNA sequence variants. Nat Genet. 2007;39:571–572. author reply 572. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

