Mitohormesis: Promoting Health and Lifespan by Increased Levels of Reactive Oxygen Species (ROS)
- PMID: 24910588
- PMCID: PMC4036400
- DOI: 10.2203/dose-response.13-035.Ristow
Mitohormesis: Promoting Health and Lifespan by Increased Levels of Reactive Oxygen Species (ROS)
Abstract
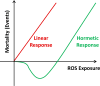
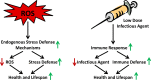
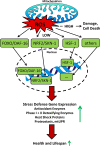
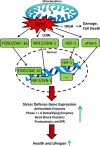
Increasing evidence indicates that reactive oxygen species (ROS), consisting of superoxide, hydrogen peroxide, and multiple others, do not only cause oxidative stress, but rather may function as signaling molecules that promote health by preventing or delaying a number of chronic diseases, and ultimately extend lifespan. While high levels of ROS are generally accepted to cause cellular damage and to promote aging, low levels of these may rather improve systemic defense mechanisms by inducing an adaptive response. This concept has been named mitochondrial hormesis or mitohormesis. We here evaluate and summarize more than 500 publications from current literature regarding such ROS-mediated low-dose signaling events, including calorie restriction, hypoxia, temperature stress, and physical activity, as well as signaling events downstream of insulin/IGF-1 receptors, AMP-dependent kinase (AMPK), target-of-rapamycin (TOR), and lastly sirtuins to culminate in control of proteostasis, unfolded protein response (UPR), stem cell maintenance and stress resistance. Additionally, consequences of interfering with such ROS signals by pharmacological or natural compounds are being discussed, concluding that particularly antioxidants are useless or even harmful.
Figures

References
-
- Adachi H, Fujiwara Y, Ishii N. Effects of oxygen on protein carbonyl and aging in Caenorhabditis elegans mutants with long (age-1) and short (mev-1) life spans. J Gerontol A Biol Sci Med Sci. 1998;53:B240–4. - PubMed
-
- Agarwal S, Sharma S, Agrawal V, Roy N. Caloric restriction augments ROS defense in S. cerevisiae by a Sir2p independent mechanism. Free Radic Res. 2005;39:55–62. - PubMed
-
- Akimoto T, Pohnert SC, Li P, Zhang M, Gumbs C, Rosenberg PB, Williams RS, Yan Z. Exercise stimulates Pgc-1alpha transcription in skeletal muscle through activation of the p38 MAPK pathway. J Biol Chem. 2005;280:19587–93. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

