Bone morphogenetic protein 2: a potential new player in the pathogenesis of diabetic retinopathy
- PMID: 24910902
- PMCID: PMC4122600
- DOI: 10.1016/j.exer.2014.05.012
Bone morphogenetic protein 2: a potential new player in the pathogenesis of diabetic retinopathy
Abstract
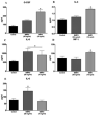
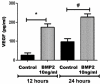
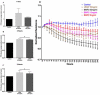
Diabetic retinopathy (DR) is one of the most common complications of diabetes mellitus. Vision loss in DR principally occurs due to breakdown of the blood-retinal barrier (BRB), leading to macular edema, retinal detachment and inner retinal and vitreous hemorrhage. Several growth factors have been shown to play crucial role in the development of these vascular changes; however, the cellular and molecular mechanisms of DR are not yet fully revealed. In the current study we investigated the role of bone morphogenetic protein-2 (BMP2) in DR. We examined the changes in the protein levels of BMP2 in human vitreous and retina in addition to the mouse retina of streptozotocin-induced diabetes. To detect the source of BMP2 during diabetes, human retinal endothelial cells (hRECs) were subjected to high glucose (HG) for 5 days and levels of BMP2 protein were analyzed in conditioned media of these cells relative to control. We also evaluated the effect of BMP2 on the levels of VEGF in cultured rat Müller cells (rMC1). In addition, we tested the pro-inflammatory effects of BMP2 by examining its effect on leukocyte adhesion to cultured hRECs, and levels of adhesion molecules and cytokines production. Finally, the effect of different concentrations of BMP2 on permeability of confluent monolayer of hRECs was evaluated using FITC-Dextran flux permeability assay and by measuring Transcellular Electrical Resistance (TER) using Electric Cell-substrate Impedance Sensing (ECIS). Our results show, for the first time, the up-regulation of BMP2 in diabetic human and mouse retinas in addition to its detection in vitreous of patients with proliferative DR (72 ± 7 pg/ml). In vitro, hRECs showed upregulation of BMP2 in HG conditions suggesting that these cells are a potential source of BMP2 in diabetic conditions. Furthermore, BMP2 induced VEGF secretion by Müller cells in-vitro; and showed a dose response in increasing permeability of cultured hRECs. Meanwhile, BMP2 pro-inflammatory effects were recognized by its ability to induce leukocyte adhesion to the hRECs, intercellular adhesion molecule-1 (ICAM-1) and upregulation of interleukin-6 and 8 (IL-6 and IL-8). These results show that BMP2 could be a contributing growth factor to the development of microvascular dysfunction during DR via enhancing both pro-angiogenic and inflammatory pathways. Our findings suggest BMP2 as a potential therapeutic target to prevent/treat DR.
Keywords: BMP 2; Müller cells; VEGF; blood–retinal barrier; diabetic retinopathy; inflammation; leukocyte adhesion; retinal endothelial cells.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures


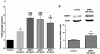
References
-
- Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, Pasquale LR, Thieme H, Iwamoto MA, Park JE, Nguyen HV, Aiello LM, Ferrara N, King GL. VASCULAR ENDOTHELIAL GROWTH-FACTOR IN OCULAR FLUID OF PATIENTS WITH DIABETIC-RETINOPATHY AND OTHER RETINAL DISORDERS. New England Journal of Medicine. 1994;331:1480–1487. - PubMed
-
- Akeel S, El-awady A, Hussein K, El-Refaey M, Elsalanty M, Sharawy M, Al-Shabrawey M. Recombinant bone morphogenetic protein-2 induces upregulation of vascular endothelial growth factor and interleukin 6 in human preosteoblasts: Role of reactive oxygen species. Archives of Oral Biology. 2012;57:445–452. - PubMed
-
- Al-Shabrawey M, Mussell R, Kahook K, Tawfik A, Eladl M, Sarthy V, Nussbaum J, El-Marakby A, Park SY, Gurel Z, Sheibani N, Maddipati KR. Increased Expression and Activity of 12-Lipoxygenase in Oxygen-Induced Ischemic Retinopathy and Proliferative Diabetic Retinopathy Implications in Retinal Neovascularization. Diabetes. 2011;60:614–624. - PMC - PubMed
-
- Bandello F, Lattanzio R, Zucchiatti I, Del Turco C. Pathophysiology and treatment of diabetic retinopathy. Acta Diabetologica. 2013;50:1–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

