Fucoidan can function as an adjuvant in vivo to enhance dendritic cell maturation and function and promote antigen-specific T cell immune responses
- PMID: 24911024
- PMCID: PMC4049775
- DOI: 10.1371/journal.pone.0099396
Fucoidan can function as an adjuvant in vivo to enhance dendritic cell maturation and function and promote antigen-specific T cell immune responses
Abstract
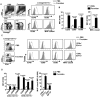
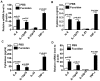
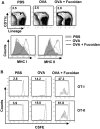
Fucoidan, a sulfated polysaccharide purified from brown algae, has a variety of immune-modulation effects, including promoting antigen uptake and enhancing anti-viral and anti-tumor effects. However, the effect of fucoidan in vivo, especially its adjuvant effect on in vivo anti-tumor immune responses, was not fully investigated. In this study, we investigated the effect of fucoidan on the function of spleen dendritic cells (DCs) and its adjuvant effect in vivo. Systemic administration of fucoidan induced up-regulation of CD40, CD80 and CD86 expression and production of IL-6, IL-12 and TNF-α in spleen cDCs. Fucoidan also promoted the generation of IFN-γ-producing Th1 and Tc1 cells in an IL-12-dependent manner. When used as an adjuvant in vivo with ovalbumin (OVA) antigen, fucoidan promoted OVA-specific antibody production and primed IFN-γ production in OVA-specific T cells. Moreover, fucoidan enhanced OVA-induced up-regulation of MHC class I and II on spleen cDCs and strongly prompted the proliferation of OVA-specific CD4 and CD8 T cells. Finally, OVA immunization with fucoidan as adjuvant protected mice from the challenge with B16-OVA tumor cells. Taken together, these results suggest that fucoidan can function as an adjuvant to induce Th1 immune response and CTL activation, which may be useful in tumor vaccine development.
Conflict of interest statement
Figures



References
-
- Banchereau J, Steinman RM (1998) Dendritic cells and the control of immunity. Nature 392: 245–252. - PubMed
-
- Banchereau J, Palucka AK (2005) Dendritic cells as therapeutic vaccines against cancer. Nature reviews Immunology 5: 296–306. - PubMed
-
- Pooley JL, Heath WR, Shortman K (2001) Cutting edge: intravenous soluble antigen is presented to CD4 T cells by CD8- dendritic cells, but cross-presented to CD8 T cells by CD8+ dendritic cells. Journal of immunology 166: 5327–5330. - PubMed
-
- Shortman K, Heath WR (2010) The CD8+ dendritic cell subset. Immunological reviews 234: 18–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

