Immune activation alters cellular and humoral responses to yellow fever 17D vaccine
- PMID: 24911151
- PMCID: PMC4071376
- DOI: 10.1172/JCI75429
Immune activation alters cellular and humoral responses to yellow fever 17D vaccine
Erratum in
- J Clin Invest. 2014 Oct 1;124(10):4669. Gaucher, Denis [Added]
Abstract
Background: Defining the parameters that modulate vaccine responses in African populations will be imperative to design effective vaccines for protection against HIV, malaria, tuberculosis, and dengue virus infections. This study aimed to evaluate the contribution of the patient-specific immune microenvironment to the response to the licensed yellow fever vaccine 17D (YF-17D) in an African cohort.
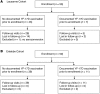
Methods: We compared responses to YF-17D in 50 volunteers in Entebbe, Uganda, and 50 volunteers in Lausanne, Switzerland. We measured the CD8+ T cell and B cell responses induced by YF-17D and correlated them with immune parameters analyzed by flow cytometry prior to vaccination.
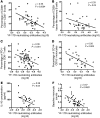
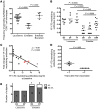
Results: We showed that YF-17D-induced CD8+ T cell and B cell responses were substantially lower in immunized individuals from Entebbe compared with immunized individuals from Lausanne. The impaired vaccine response in the Entebbe cohort associated with reduced YF-17D replication. Prior to vaccination, we observed higher frequencies of exhausted and activated NK cells, differentiated T and B cell subsets and proinflammatory monocytes, suggesting an activated immune microenvironment in the Entebbe volunteers. Interestingly, activation of CD8+ T cells and B cells as well as proinflammatory monocytes at baseline negatively correlated with YF-17D-neutralizing antibody titers after vaccination. Additionally, memory T and B cell responses in preimmunized volunteers exhibited reduced persistence in the Entebbe cohort but were boosted by a second vaccination.
Conclusion: Together, these results demonstrate that an activated immune microenvironment prior to vaccination impedes efficacy of the YF-17D vaccine in an African cohort and suggest that vaccine regimens may need to be boosted in African populations to achieve efficient immunity.
Trial registration: Registration is not required for observational studies.
Funding: This study was funded by Canada's Global Health Research Initiative, Defense Threat Reduction Agency, National Institute of Allergy and Infectious Diseases, Bill & Melinda Gates Foundation, and United States Agency for International Development.
Figures





References
-
- Monath TP, Barrett AD. Pathogenesis and pathophysiology of yellow fever. Adv Virus Res. 2003;60:343–395. - PubMed
-
- Niedrig M, Lademann M, Emmerich P, Lafrenz M. Assessment of IgG antibodies against yellow fever virus after vaccination with 17D by different assays: neutralization test, haemagglutination inhibition test, immunofluorescence assay and ELISA. Trop Med Int Health. 1999;4(12):867–871. doi: 10.1046/j.1365-3156.1999.00496.x. - DOI - PubMed
-
- Monath TP, Cetron MS, Teuwen DE. Yellow fever vaccine. In: Plotkin SA, Orenstein WA, Offit PA, eds. Vaccines. 5th ed. Philadelphia, Pennsylvania, USA: Saunders Elsevier; 2008:959–1055.
-
- Staples JE, Gershman M, Fischer M. Yellow fever vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2010;59(RR-7):1–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

