Proteotoxicity is not the reason for the dependence of cancer cells on the major chaperone Hsp70
- PMID: 24911412
- PMCID: PMC4111684
- DOI: 10.4161/cc.29296
Proteotoxicity is not the reason for the dependence of cancer cells on the major chaperone Hsp70
Abstract
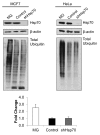
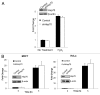
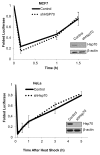
Several years ago a hypothesis was proposed that the survival of cancer cells depend on elevated expression of molecular chaperones because these cells are prone to proteotoxic stress. A critical prediction of this hypothesis is that depletion of chaperones in cancer cells should lead to proteotoxicity. Here, using the major chaperone Hsp70 as example, we demonstrate that its depletion does not trigger proteotoxic stress, thus refuting the model. Accordingly, other functions of chaperones, e.g., their role in cell signaling, might define the requirements for chaperones in cancer cells, which is critical for rational targeting Hsp70 in cancer treatment.
Keywords: Hsp70; chaperone; non-oncogene addiction; proteotoxic stress; rational drug targeting.
Figures

Similar articles
-
Hsp70 in cancer: back to the future.Oncogene. 2015 Aug 6;34(32):4153-61. doi: 10.1038/onc.2014.349. Epub 2014 Oct 27. Oncogene. 2015. PMID: 25347739 Free PMC article. Review.
-
Unique integrated stress response sensors regulate cancer cell susceptibility when Hsp70 activity is compromised.Elife. 2021 Jun 28;10:e64977. doi: 10.7554/eLife.64977. Elife. 2021. PMID: 34180400 Free PMC article.
-
Inducible HSP70 is critical in preventing the aggregation and enhancing the processing of PMP22.ASN Neuro. 2015 Feb 18;7(1):1759091415569909. doi: 10.1177/1759091415569909. Print 2015 Jan-Feb. ASN Neuro. 2015. PMID: 25694550 Free PMC article.
-
OLA1 protects cells in heat shock by stabilizing HSP70.Cell Death Dis. 2013 Feb 14;4(2):e491. doi: 10.1038/cddis.2013.23. Cell Death Dis. 2013. PMID: 23412384 Free PMC article.
-
Microbial molecular chaperones.Adv Microb Physiol. 2001;44:93-140. doi: 10.1016/s0065-2911(01)44012-4. Adv Microb Physiol. 2001. PMID: 11407116 Review.
Cited by
-
Cytoplasmic proteotoxicity regulates HRI-dependent phosphorylation of eIF2α via the Hsp70-Bag3 module.iScience. 2022 Apr 22;25(5):104282. doi: 10.1016/j.isci.2022.104282. eCollection 2022 May 20. iScience. 2022. PMID: 35573186 Free PMC article.
-
HSP70s in Breast Cancer: Promoters of Tumorigenesis and Potential Targets/Tools for Therapy.Cells. 2021 Dec 7;10(12):3446. doi: 10.3390/cells10123446. Cells. 2021. PMID: 34943954 Free PMC article. Review.
-
Hsp70 in cancer: back to the future.Oncogene. 2015 Aug 6;34(32):4153-61. doi: 10.1038/onc.2014.349. Epub 2014 Oct 27. Oncogene. 2015. PMID: 25347739 Free PMC article. Review.
-
Therapeutic targeting of BAG3: considering its complexity in cancer and heart disease.J Clin Invest. 2021 Aug 16;131(16):e149415. doi: 10.1172/JCI149415. J Clin Invest. 2021. PMID: 34396980 Free PMC article. Review.
References
-
- Santagata S, Hu R, Lin NU, Mendillo ML, Collins LC, Hankinson SE, Schnitt SJ, Whitesell L, Tamimi RM, Lindquist S, et al. High levels of nuclear heat-shock factor 1 (HSF1) are associated with poor prognosis in breast cancer. Proc Natl Acad Sci U S A. 2011;108:18378–83. doi: 10.1073/pnas.1115031108. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
