Vascular pathobiology in chronic liver disease and cirrhosis - current status and future directions
- PMID: 24911462
- PMCID: PMC4346093
- DOI: 10.1016/j.jhep.2014.05.047
Vascular pathobiology in chronic liver disease and cirrhosis - current status and future directions
Abstract
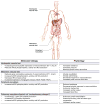
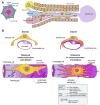
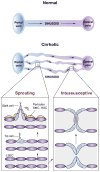
Chronic liver disease is associated with remarkable alterations in the intra- and extrahepatic vasculature. Because of these changes, the fields of liver vasculature and portal hypertension have recently become closely integrated within the broader vascular biology discipline. As developments in vascular biology have evolved, a deeper understanding of vascular processes has led to a better understanding of the mechanisms of the dynamic vascular changes associated with portal hypertension and chronic liver disease. In this context, hepatic vascular cells, such as sinusoidal endothelial cells and pericyte-like hepatic stellate cells, are closely associated with one another, where they have paracrine and autocrine effects on each other and themselves. These cells play important roles in the pathogenesis of liver fibrosis/cirrhosis and portal hypertension. Further, a variety of signaling pathways have recently come to light. These include growth factor pathways involving cytokines such as transforming growth factor β, platelet derived growth factor, and others as well as a variety of vasoactive peptides and other molecules. An early and consistent feature of liver injury is the development of an increase in intra-hepatic resistance; this is associated with changes in hepatic vascular cells and their signaling pathway that cause portal hypertension. A critical concept is that this process aggregates signals to the extrahepatic circulation, causing derangement in this system's cells and signaling pathways, which ultimately leads to the collateral vessel formation and arterial vasodilation in the splanchnic and systemic circulation, which by virtue of the hydraulic derivation of Ohm's law (pressure = resistance × flow), worsens portal hypertension. This review provides a detailed review of the current status and future direction of the basic biology of portal hypertension with a focus on the physiology, pathophysiology, and signaling of cells within the liver, as well as those in the mesenteric vascular circulation. Translational implications of recent research and the future directions that it points to are also highlighted.
Keywords: Endothelial cell; Pericyte; Pressure; Resistance; Sinusoid; Therapy.
Copyright © 2014 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.
Figures

Similar articles
-
Pathophysiology of portal hypertension.Clin Liver Dis. 2014 May;18(2):281-91. doi: 10.1016/j.cld.2013.12.001. Epub 2014 Feb 25. Clin Liver Dis. 2014. PMID: 24679494 Free PMC article. Review.
-
Endothelial dysfunction in the regulation of cirrhosis and portal hypertension.Liver Int. 2012 Feb;32(2):199-213. doi: 10.1111/j.1478-3231.2011.02579.x. Epub 2011 Jul 5. Liver Int. 2012. PMID: 21745318 Free PMC article. Review.
-
Pathophysiology and a Rational Basis of Therapy.Dig Dis. 2015;33(4):508-14. doi: 10.1159/000374099. Epub 2015 Jul 6. Dig Dis. 2015. PMID: 26159267
-
Biology of portal hypertension.Hepatol Int. 2018 Feb;12(Suppl 1):11-23. doi: 10.1007/s12072-017-9826-x. Epub 2017 Oct 26. Hepatol Int. 2018. PMID: 29075990 Free PMC article. Review.
-
[Pathogenesis of portal hypertension].Rev Invest Clin. 2005 Jul-Aug;57(4):596-607. Rev Invest Clin. 2005. PMID: 16315644 Review. Spanish.
Cited by
-
Modulation of vascular contraction via soluble guanylate cyclase signaling in a novel ex vivo method using rat precision-cut liver slices.Pharmacol Res Perspect. 2021 May;9(3):e00768. doi: 10.1002/prp2.768. Pharmacol Res Perspect. 2021. PMID: 34014044 Free PMC article.
-
Initial Fluid Resuscitation Following Adjusted Body Weight Dosing in Sepsis and Septic Shock.J Crit Care Med (Targu Mures). 2019 Nov 27;5(4):130-135. doi: 10.2478/jccm-2019-0025. eCollection 2019 Oct. J Crit Care Med (Targu Mures). 2019. PMID: 31915718 Free PMC article.
-
β-Arrestin2 is a critical component of the GPCR-eNOS signalosome.Proc Natl Acad Sci U S A. 2020 May 26;117(21):11483-11492. doi: 10.1073/pnas.1922608117. Epub 2020 May 13. Proc Natl Acad Sci U S A. 2020. PMID: 32404425 Free PMC article.
-
Liver sinusoidal endothelial cells (LSECs) modifications in patients with chronic hepatitis C.Sci Rep. 2019 Jun 19;9(1):8760. doi: 10.1038/s41598-019-45114-1. Sci Rep. 2019. PMID: 31217430 Free PMC article. Clinical Trial.
-
Cellular distribution of injected PLGA-nanoparticles in the liver.Nanomedicine. 2016 Jul;12(5):1365-74. doi: 10.1016/j.nano.2016.01.013. Epub 2016 Mar 4. Nanomedicine. 2016. PMID: 26961463 Free PMC article.
References
-
- Wisse E, De Zanger RB, Jacobs R, McCuskey RS. Scanning electron microscope observations on the structure of portal veins, sinusoids and central veins in rat liver. Scanning Microsc. 1983:1441–1452. - PubMed
-
- Bhunchet E, Fujieda K. Capillarization and venularization of hepatic sinusoids in porcine serum-induced rat liver fibrosis: a mechanism to maintain liver blood flow. Hepatology. 1993;18:1450–1458. - PubMed
-
- Shah V, Toruner M, Haddad F, Cadelina G, Papapetropoulos A, Choo K, et al. Impaired endothelial nitric oxide synthase activity associated with enhanced caveolin binding in experimental cirrhosis in the rat. Gastroenterology. 1999;117:1222–1228. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

