Internalization of isolated functional mitochondria: involvement of macropinocytosis
- PMID: 24912369
- PMCID: PMC4190914
- DOI: 10.1111/jcmm.12316
Internalization of isolated functional mitochondria: involvement of macropinocytosis
Abstract
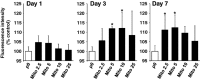
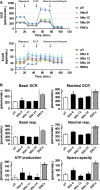
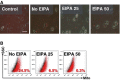
In eukaryotic cells, mitochondrial dysfunction is associated with a variety of human diseases. Delivery of exogenous functional mitochondria into damaged cells has been proposed as a mechanism of cell transplant and physiological repair for damaged tissue. We here demonstrated that isolated mitochondria can be transferred into homogeneic and xenogeneic cells by simple co-incubation using genetically labelled mitochondria, and elucidated the mechanism and the effect of direct mitochondrial transfer. Intracellular localization of exogenous mitochondria was confirmed by PCR, real-time PCR, live fluorescence imaging, three-dimensional reconstruction imaging, continuous time-lapse microscopic observation, flow cytometric analysis and immunoelectron microscopy. Isolated homogeneic mitochondria were transferred into human uterine endometrial gland-derived mesenchymal cells in a dose-dependent manner. Moreover, mitochondrial transfer rescued the mitochondrial respiratory function and improved the cellular viability in mitochondrial DNA-depleted cells and these effects lasted several days. Finally, we discovered that mitochondrial internalization involves macropinocytosis. In conclusion, these data support direct transfer of exogenous mitochondria as a promising approach for the treatment of various diseases.
Keywords: macropinocytosis; mitochondrial transfer; rho0 cells.
© 2014 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Figures




Similar articles
-
Direct human mitochondrial transfer: a novel concept based on the endosymbiotic theory.Transplant Proc. 2014 May;46(4):1233-6. doi: 10.1016/j.transproceed.2013.11.133. Transplant Proc. 2014. PMID: 24815168
-
Xenogenic transfer of isolated murine mitochondria into human rho0 cells can improve respiratory function.Rejuvenation Res. 2007 Dec;10(4):561-70. doi: 10.1089/rej.2007.0575. Rejuvenation Res. 2007. PMID: 18069915
-
TAT-dextran-mediated mitochondrial transfer enhances recovery from models of reperfusion injury in cultured cardiomyocytes.J Cell Mol Med. 2020 May;24(9):5007-5020. doi: 10.1111/jcmm.15120. Epub 2020 Mar 25. J Cell Mol Med. 2020. PMID: 32212298 Free PMC article.
-
From Cell Entry to Engraftment of Exogenous Mitochondria.Int J Mol Sci. 2020 Jul 15;21(14):4995. doi: 10.3390/ijms21144995. Int J Mol Sci. 2020. PMID: 32679802 Free PMC article. Review.
-
Mitotherapy as a Novel Therapeutic Strategy for Mitochondrial Diseases.Curr Mol Pharmacol. 2020;13(1):41-49. doi: 10.2174/1874467212666190920144115. Curr Mol Pharmacol. 2020. PMID: 31345157 Review.
Cited by
-
The Functions, Methods, and Mobility of Mitochondrial Transfer Between Cells.Front Oncol. 2021 May 10;11:672781. doi: 10.3389/fonc.2021.672781. eCollection 2021. Front Oncol. 2021. PMID: 34041035 Free PMC article. Review.
-
Mitochondrial transplantation for therapeutic use.Clin Transl Med. 2016 Mar;5(1):16. doi: 10.1186/s40169-016-0095-4. Epub 2016 Apr 29. Clin Transl Med. 2016. PMID: 27130633 Free PMC article. Review.
-
OPA1 regulates respiratory supercomplexes assembly: The role of mitochondrial swelling.Mitochondrion. 2020 Mar;51:30-39. doi: 10.1016/j.mito.2019.11.006. Epub 2019 Dec 20. Mitochondrion. 2020. PMID: 31870826 Free PMC article.
-
Emerging roles of mitochondria in animal regeneration.Cell Regen. 2023 May 5;12(1):14. doi: 10.1186/s13619-023-00158-7. Cell Regen. 2023. PMID: 37142814 Free PMC article. Review.
-
Platelet-derived microparticles provoke chronic lymphocytic leukemia malignancy through metabolic reprogramming.Front Immunol. 2023 Jun 27;14:1207631. doi: 10.3389/fimmu.2023.1207631. eCollection 2023. Front Immunol. 2023. PMID: 37441073 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

