Inhibitory effects of prostaglandin E2 on collagen synthesis and cell proliferation in human stellate cells from pancreatic head adenocarcinoma
- PMID: 24912820
- PMCID: PMC4084579
- DOI: 10.1186/1471-2407-14-413
Inhibitory effects of prostaglandin E2 on collagen synthesis and cell proliferation in human stellate cells from pancreatic head adenocarcinoma
Abstract
Background: Several studies have described an increased cyclooxygenase-2 (COX-2) expression in pancreatic cancer, but the role of COX-2 in tumour development and progression is not clear. The aim of the present study was to examine expression of COX-2 in cancer cells and stromal cells in pancreatic cancer specimens, and to explore the role of PGE2 in pancreatic stellate cell proliferation and collagen synthesis.
Methods: Immunohistochemistry and immunofluorescence was performed on slides from whole sections of tissue blocks using antibodies against COX-2 and α-smooth muscle actin (αSMA). Pancreatic stellate cells (PSC) were isolated from surgically resected tumour tissue by the outgrowth method. Cells were used between passages 4 and 8. Collagen synthesis was determined by [(3)H]-proline incorporation, or by enzyme immunoassay measurement of collagen C-peptide. DNA synthesis was measured by incorporation of [(3)H]-thymidine in DNA. Cyclic AMP (cAMP) was determined by radioimmunoassay. Collagen 1A1 mRNA was determined by RT-qPCR.
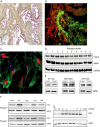
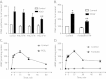
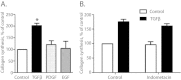
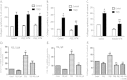
Results: Immunohistochemistry staining showed COX-2 in pancreatic carcinoma cells, but not in stromal cells. All tumours showed positive staining for αSMA in the fibrotic stroma. Cultured PSC expressed COX-2, which could be further induced by interleukin-1β (IL-1β), epidermal growth factor (EGF), thrombin, and PGE2, but not by transforming growth factor-β1 (TGFβ). Indirect coculture with the adenocarcinoma cell line BxPC-3, but not HPAFII or Panc-1, induced COX-2 expression in PSC. Treatment of PSC with PGE2 strongly stimulated cAMP accumulation, mediated by EP2 receptors, and also stimulated phosphorylation of extracellular signal-regulated kinase (ERK). Treatment of PSC with PGE2 or forskolin suppressed both TGFβ-stimulated collagen synthesis and PDGF-stimulated DNA synthesis.
Conclusions: The present results show that COX-2 is mainly produced in carcinoma cells and suggest that the cancer cells are the main source of PGE2 in pancreatic tumours. PGE2 exerts a suppressive effect on proliferation and fibrogenesis in pancreatic stellate cells. These effects of PGE2 are mediated by the cAMP pathway and suggest a role of EP2 receptors.
Figures



Similar articles
-
Prostaglandin E2 regulates pancreatic stellate cell activity via the EP4 receptor.Pancreas. 2013 Apr;42(3):467-74. doi: 10.1097/MPA.0b013e318264d0f8. Pancreas. 2013. PMID: 23090667 Free PMC article.
-
Direct growth-inhibitory effects of prostaglandin E2 in pancreatic cancer cells in vitro through an EP4/PKA-mediated mechanism.Surgery. 2017 Jun;161(6):1570-1578. doi: 10.1016/j.surg.2016.12.037. Epub 2017 Feb 20. Surgery. 2017. PMID: 28222855 Free PMC article.
-
The TGFβ-SMAD3 pathway inhibits IL-1α induced interactions between human pancreatic stellate cells and pancreatic carcinoma cells and restricts cancer cell migration.J Exp Clin Cancer Res. 2016 Jul 29;35(1):122. doi: 10.1186/s13046-016-0400-5. J Exp Clin Cancer Res. 2016. PMID: 27473228 Free PMC article.
-
Pancreatic stellate cell: Update on molecular investigations and clinical translation in pancreatic cancer.Int J Cancer. 2025 May 1;156(9):1672-1685. doi: 10.1002/ijc.35326. Epub 2025 Jan 18. Int J Cancer. 2025. PMID: 39825771 Review.
-
Heterogeneous Pancreatic Stellate Cells Are Powerful Contributors to the Malignant Progression of Pancreatic Cancer.Front Cell Dev Biol. 2021 Dec 20;9:783617. doi: 10.3389/fcell.2021.783617. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34988078 Free PMC article. Review.
Cited by
-
Lysophosphatidylcholine-induced mitochondrial fission contributes to collagen production in human cardiac fibroblasts.J Lipid Res. 2019 Sep;60(9):1573-1589. doi: 10.1194/jlr.RA119000141. Epub 2019 Jul 30. J Lipid Res. 2019. PMID: 31363041 Free PMC article.
-
Pancreatic stellate cell-induced gemcitabine resistance in pancreatic cancer is associated with LDHA- and MCT4-mediated enhanced glycolysis.Cancer Cell Int. 2023 Jan 19;23(1):9. doi: 10.1186/s12935-023-02852-7. Cancer Cell Int. 2023. PMID: 36658582 Free PMC article.
-
Cinobufacini Inhibits the Development of Pancreatic Cancer Cells through the TGFβ/Smads Pathway of Pancreatic Stellate Cells.Evid Based Complement Alternat Med. 2022 Jun 30;2022:3719857. doi: 10.1155/2022/3719857. eCollection 2022. Evid Based Complement Alternat Med. 2022. Retraction in: Evid Based Complement Alternat Med. 2023 Dec 13;2023:9843430. doi: 10.1155/2023/9843430. PMID: 35815263 Free PMC article. Retracted.
-
Functional heterogeneity in tumor-derived human pancreatic stellate cells: Differential expression of HGF and implications for mitogenic signaling and migration in pancreatic cancer cells.Oncotarget. 2017 May 11;8(42):71672-71684. doi: 10.18632/oncotarget.17800. eCollection 2017 Sep 22. Oncotarget. 2017. PMID: 29069737 Free PMC article.
-
Distinct Metalloproteinase Expression and Functions in Systemic Sclerosis and Fibrosis: What We Know and the Potential for Intervention.Front Physiol. 2021 Aug 27;12:727451. doi: 10.3389/fphys.2021.727451. eCollection 2021. Front Physiol. 2021. PMID: 34512395 Free PMC article. Review.
References
-
- Krejs GJ. Pancreatic cancer: epidemiology and risk factors. Dig Dis. 2010;28(2):355–358. - PubMed
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. - PubMed
-
- Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362(17):1605–1617. - PubMed
-
- Chu GC, Kimmelman AC, Hezel AF, DePinho RA. Stromal biology of pancreatic cancer. J Cell Biochem. 2007;101(4):887–907. - PubMed
-
- Apte MV, Park S, Phillips PA, Santucci N, Goldstein D, Kumar RK, Ramm GA, Buchler M, Friess H, McCarroll JA, Keogh G, Merrett N, Pirola R, Wilson JS. Desmoplastic reaction in pancreatic cancer: role of pancreatic stellate cells. Pancreas. 2004;29(3):179–187. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

