Neutrophils recruited by chemoattractants in vivo induce microvascular plasma protein leakage through secretion of TNF
- PMID: 24913232
- PMCID: PMC4076577
- DOI: 10.1084/jem.20132413
Neutrophils recruited by chemoattractants in vivo induce microvascular plasma protein leakage through secretion of TNF
Abstract
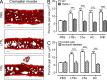
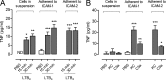
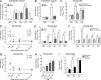
Microvascular plasma protein leakage is an essential component of the inflammatory response and serves an important function in local host defense and tissue repair. Mediators such as histamine and bradykinin act directly on venules to increase the permeability of endothelial cell (EC) junctions. Neutrophil chemoattractants also induce leakage, a response that is dependent on neutrophil adhesion to ECs, but the underlying mechanism has proved elusive. Through application of confocal intravital microscopy to the mouse cremaster muscle, we show that neutrophils responding to chemoattractants release TNF when in close proximity of EC junctions. In vitro, neutrophils adherent to ICAM-1 or ICAM-2 rapidly released TNF in response to LTB4, C5a, and KC. Further, in TNFR(-/-) mice, neutrophils accumulated normally in response to chemoattractants administered to the cremaster muscle or dorsal skin, but neutrophil-dependent plasma protein leakage was abolished. Similar results were obtained in chimeric mice deficient in leukocyte TNF. A locally injected TNF blocking antibody was also able to inhibit neutrophil-dependent plasma leakage, but had no effect on the response induced by bradykinin. The results suggest that TNF mediates neutrophil-dependent microvascular leakage. This mechanism may contribute to the effects of TNF inhibitors in inflammatory diseases and indicates possible applications in life-threatening acute edema.
© 2014 Finsterbusch.
Figures

References
-
- Arfors K.E., Lundberg C., Lindbom L., Lundberg K., Beatty P.G., Harlan J.M. 1987. A monoclonal antibody to the membrane glycoprotein complex CD18 inhibits polymorphonuclear leukocyte accumulation and plasma leakage in vivo. Blood. 69:338–340 - PubMed
-
- Colom B., Poitelon Y., Huang W., Woodfin A., Averill S., Del Carro U., Zambroni D., Brain S.D., Perretti M., Ahluwalia A., et al. 2012. Schwann cell-specific JAM-C-deficient mice reveal novel expression and functions for JAM-C in peripheral nerves. FASEB J. 26:1064–1076 10.1096/fj.11-196220 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

