A cisplatin-resistant head and neck cancer cell line with cytoplasmic p53(mut) exhibits ATP-binding cassette transporter upregulation and high glutathione levels
- PMID: 24913304
- PMCID: PMC11823790
- DOI: 10.1007/s00432-014-1727-y
A cisplatin-resistant head and neck cancer cell line with cytoplasmic p53(mut) exhibits ATP-binding cassette transporter upregulation and high glutathione levels
Abstract
Purpose: Head and neck squamous cell carcinoma (HNSCC) cell lines with cytoplasmically sequestered mutant p53 (p53(mut_c)) are frequently more resistant to cisplatin (CDDP) than cells with mutant but nuclear p53 (p53(mut_n)). The aim of the study was to identify underlying mechanisms implicated in CDDP resistance of HNSCC cells carrying cytoplasmic p53(mut).
Methods: Microarray analysis, quantitative reverse transcription polymerase chain reaction, Western blot analysis and immunocytochemistry were used to identify and evaluate candidate genes involved in CDDP resistance of p53(mut_c) cells. RNAi knockdown or treatment with inhibitors together with flow cytometry-based methods was used for functional assessment of the identified candidate genes. Cellular metabolic activity was assessed with the XTT assay, and the redox capacity of cells was evaluated by measuring cellular glutathione (GSH) levels.
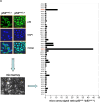
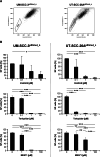
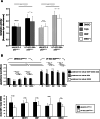
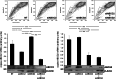
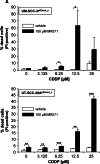
Results: Upregulation of ABCC2 and ABCG2 transporters was observed in CDDP-resistant p53(mut_c) HNSCC cells. Furthermore, p53(mut_c) cells exhibited a pronounced side population that could be suppressed by RNAi knockdown of ABCG2 as well as treatment with the ATP-binding-cassette transporter inhibitors imatinib, MK571 and tariquidar. Metabolic activity and cellular GSH levels were higher in CDDP-resistant p53(mut_c) cells, consistent with a higher capacity to fend off cytotoxic oxidative effects such as those caused by CDDP treatment. Finally, ABCC2/G2 inhibition of HNSCC cells with MK571 markedly enhanced CDDP sensitivity of HNSCC cells.
Conclusions: The observations in this study point to a major role of p53(mut_c) in conferring a stem cell like phenotype to HNSCC cells that is associated with ABCC2/G2 overexpression, high GSH and metabolic activity levels as well as CDDP resistance.
Conflict of interest statement
All authors have no conflict of interest.
Figures


References
-
- Akiyama M (2010) ABCA12 mutations and autosomal recessive congenital ichthyosis: a review of genotype/phenotype correlations and of pathogenetic concepts. Hum Mutat 31:1090–1096 - PubMed
-
- Brendel C, Scharenberg C, Dohse M, Robey RW, Bates SE, Shukla S, Ambudkar SV, Wang Y, Wennemuth G, Burchert A, Boudriot U, Neubauer A (2007) Imatinib mesylate and nilotinib (AMN107) exhibit high-affinity interaction with ABCG2 on primitive hematopoietic stem cells. Leukemia 21:1267–1275 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

