The node of Ranvier in CNS pathology
- PMID: 24913350
- PMCID: PMC4102831
- DOI: 10.1007/s00401-014-1305-z
The node of Ranvier in CNS pathology
Abstract
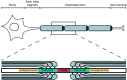
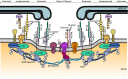
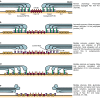
Healthy nodes of Ranvier are crucial for action potential propagation along myelinated axons, both in the central and in the peripheral nervous system. Surprisingly, the node of Ranvier has often been neglected when describing CNS disorders, with most pathologies classified simply as being due to neuronal defects in the grey matter or due to oligodendrocyte damage in the white matter. However, recent studies have highlighted changes that occur in pathological conditions at the node of Ranvier, and at the associated paranodal and juxtaparanodal regions where neurons and myelinating glial cells interact. Lengthening of the node of Ranvier, failure of the electrically resistive seal between the myelin and the axon at the paranode, and retraction of myelin to expose voltage-gated K(+) channels in the juxtaparanode, may contribute to altering the function of myelinated axons in a wide range of diseases, including stroke, spinal cord injury and multiple sclerosis. Here, we review the principles by which the node of Ranvier operates and its molecular structure, and thus explain how defects at the node and paranode contribute to neurological disorders.
Figures
References
-
- Adelman JP, Bond CT, Pessia M, Maylie J. Episodic ataxia results from voltage-dependent potassium channels with altered functions. Neuron. 1995;15(6):1449–1454. - PubMed
-
- Ahn KH, Lyoo IK, Lee HK, Song IC, Oh JS, Hwang J, Kwon J, Kim MJ, Kim M, Renshaw PF. White matter hyperintensities in subjects with bipolar disorder. Psychiatry Clin Neurosci. 2004;58(5):516–521. - PubMed
-
- Athanasiu L, Mattingsdal M, Kahler AK, Brown A, Gustafsson O, Agartz I, Giegling I, Muglia P, Cichon S, Rietschel M, Pietilainen OP, Peltonen L, Bramon E, Collier D, Clair DS, Sigurdsson E, Petursson H, Rujescu D, Melle I, Steen VM, Djurovic S, Andreassen OA. Gene variants associated with schizophrenia in a Norwegian genome-wide study are replicated in a large European cohort. J Psychiatr Res. 2010;44(12):748–753. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

