Mechanisms that can promote peripheral B-cell lymphoma in ATM-deficient mice
- PMID: 24913718
- PMCID: PMC4156541
- DOI: 10.1158/2326-6066.CIR-14-0090
Mechanisms that can promote peripheral B-cell lymphoma in ATM-deficient mice
Abstract
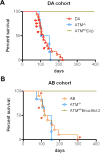
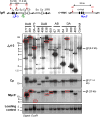
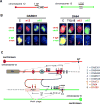
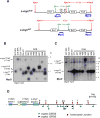
The Ataxia Telangiectasia-mutated (ATM) kinase senses DNA double-strand breaks (DSB) and facilitates their repair. In humans, ATM deficiency predisposes to B- and T-cell lymphomas, but in mice it leads only to thymic lymphomas. We tested the hypothesis that increased DSB frequency at a cellular oncogene could promote B-cell lymphoma by generating ATM-deficient mice with a V(D)J recombination target (DJβ cassette) within c-myc intron 1 ("DA" mice). We also generated ATM-deficient mice carrying an Eμ-Bcl-2 transgene (AB mice) to test whether enhanced cellular survival could promote B-cell lymphomas. About 30% of DA or AB mice and nearly 100% of mice harboring the combined genotypes (DAB mice) developed mature B-cell lymphomas. In all genotypes, B-cell tumors harbored oncogenic c-myc amplification generated by breakage-fusion-bridge (BFB) from dicentric chromosomes formed through fusion of IgH V(D)J recombination-associated DSBs on chromosome 12 to sequences downstream of c-myc on chromosome 15. AB tumors demonstrate that B lineage cells harboring spontaneous DSBs leading to IgH/c-myc dicentrics are blocked from progressing to B-cell lymphomas by cellular apoptotic responses. DA and DAB tumor translocations were strictly linked to the cassette, but occurred downstream, frequently in a 6-kb region adjacent to c-myc that harbors multiple cryptic V(D)J recombination targets, suggesting that bona fide V(D)J target sequences may activate linked cryptic targets. Our findings indicate that ATM deficiency allows IgH V(D)J recombination DSBs in developing B cells to generate dicentric translocations that, via BFB cycles, lead to c-myc-activating oncogenic translocations and amplifications in mature B cells.
©2014 American Association for Cancer Research.
Conflict of interest statement
Figures
Similar articles
-
Developmental propagation of V(D)J recombination-associated DNA breaks and translocations in mature B cells via dicentric chromosomes.Proc Natl Acad Sci U S A. 2014 Jul 15;111(28):10269-74. doi: 10.1073/pnas.1410112111. Epub 2014 Jun 30. Proc Natl Acad Sci U S A. 2014. PMID: 24982162 Free PMC article.
-
Oncogenic transformation in the absence of Xrcc4 targets peripheral B cells that have undergone editing and switching.J Exp Med. 2008 Dec 22;205(13):3079-90. doi: 10.1084/jem.20082271. Epub 2008 Dec 8. J Exp Med. 2008. PMID: 19064702 Free PMC article.
-
Long-range oncogenic activation of Igh-c-myc translocations by the Igh 3' regulatory region.Nature. 2009 Dec 10;462(7274):803-7. doi: 10.1038/nature08633. Nature. 2009. PMID: 20010689 Free PMC article.
-
Functional overlaps between XLF and the ATM-dependent DNA double strand break response.DNA Repair (Amst). 2014 Apr;16:11-22. doi: 10.1016/j.dnarep.2014.01.010. Epub 2014 Feb 20. DNA Repair (Amst). 2014. PMID: 24674624 Free PMC article. Review.
-
The Role for the DSB Response Pathway in Regulating Chromosome Translocations.Adv Exp Med Biol. 2018;1044:65-87. doi: 10.1007/978-981-13-0593-1_6. Adv Exp Med Biol. 2018. PMID: 29956292 Review.
Cited by
-
Ataxia telangiectasia: a review.Orphanet J Rare Dis. 2016 Nov 25;11(1):159. doi: 10.1186/s13023-016-0543-7. Orphanet J Rare Dis. 2016. PMID: 27884168 Free PMC article. Review.
-
Developmental propagation of V(D)J recombination-associated DNA breaks and translocations in mature B cells via dicentric chromosomes.Proc Natl Acad Sci U S A. 2014 Jul 15;111(28):10269-74. doi: 10.1073/pnas.1410112111. Epub 2014 Jun 30. Proc Natl Acad Sci U S A. 2014. PMID: 24982162 Free PMC article.
-
Chromosomal Loop Domains Direct the Recombination of Antigen Receptor Genes.Cell. 2015 Nov 5;163(4):947-59. doi: 10.1016/j.cell.2015.10.016. Epub 2015 Oct 22. Cell. 2015. PMID: 26593423 Free PMC article.
-
Transcription-associated processes cause DNA double-strand breaks and translocations in neural stem/progenitor cells.Proc Natl Acad Sci U S A. 2016 Feb 23;113(8):2258-63. doi: 10.1073/pnas.1525564113. Epub 2016 Feb 12. Proc Natl Acad Sci U S A. 2016. PMID: 26873106 Free PMC article.
-
A Rapid Embryonic Stem Cell-Based Mouse Model for B-cell Lymphomas Driven by Epstein-Barr Virus Protein LMP1.Cancer Immunol Res. 2015 Jun;3(6):641-9. doi: 10.1158/2326-6066.CIR-15-0058. Epub 2015 May 1. Cancer Immunol Res. 2015. PMID: 25934172 Free PMC article.
References
-
- Schatz DG, Swanson PC. V(D)J recombination: mechanisms of initiation. Annu Rev Genet. 2010;45:167–202. - PubMed
-
- Cobb RM, Oestreich KJ, Osipovich OA, Oltz EM. Accessibility control of V(D)J recombination. Adv Immunol. 2006;91:45–109. - PubMed
-
- Di Noia JM, Neuberger MS. Molecular mechanisms of antibody somatic hypermutation. Annu Rev Biochem. 2007;76:1–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

