Changes in QTc interval in the citalopram for agitation in Alzheimer's disease (CitAD) randomized trial
- PMID: 24914549
- PMCID: PMC4051660
- DOI: 10.1371/journal.pone.0098426
Changes in QTc interval in the citalopram for agitation in Alzheimer's disease (CitAD) randomized trial
Abstract
Background: A Food and Drug Administration (FDA) safety communication in August 2011 warned that citalopram was associated with a dose dependent risk of QT prolongation and recommended dose restriction in patients over the age of 60 but did not provide data for this age group.
Methods: CitAD was a randomized, double-masked, placebo-controlled, multicenter clinical trial for agitation in Alzheimer's disease (AD). Participants were assigned to citalopram (target dose of 30 mg/day) or placebo in a 1 ∶ 1 ratio. 186 people, 181 of whom were over the age of 60, having probable AD with clinically significant agitation were recruited from September 2009 to January 2013. After the FDA safety communication about citalopram, ECG was added to the required study procedures before enrollment and repeated at week 3 to monitor change in QTc interval. Forty-eight participants were enrolled after enhanced monitoring began.
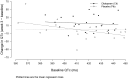
Results: Citalopram treatment was associated with a larger increase in QTc interval than placebo (difference in week 3 QTc adjusting for baseline QTc: 18.1 ms [95% CI: 6.1, 30.1]; p = 0.004). More participants in the citalopram group had an increase ≥ 30 ms from baseline to week 3 (7 in citalopram versus 1 in placebo; Fisher's exact p = 0.046), but only slightly more in the citalopram group met a gender-specific threshold for prolonged QTc (450 ms for males; 470 ms for females) at any point during follow-up (3 in citalopram versus 1 in placebo, Fisher's exact p = 0.611). One of the citalopram participants who developed prolonged QTc also displayed ventricular bigeminy. No participants in either group had a cardiovascular-related death.
Conclusion: Citalopram at 30 mg/day was associated with improvement in agitation in patients with AD but was also associated with QT prolongation.
Trial registration: ClinicalTrials.gov NCT00898807.
Conflict of interest statement
Figures
References
-
- Habib AS, Gan TJ (2008) Pro: The Food and Drug Administration Black box warning on droperidol is not justified. Anesth Analg 106: 1414–1417. - PubMed
-
- Luo S, Michler K, Johnston P, Macfarlane PW (2004) A comparison of commonly used QT correction formulae: the effect of heart rate on the QTc of normal ECGs. J Electrocardiol 37 Suppl: 81–90. - PubMed
-
- Goldenberg I, Moss AJ, Zareba W (2006) QT interval: how to measure it and what is “normal”. J Cardiovasc Electrophysiol 17: 333–336. - PubMed
-
- Al-Khatib SM, LaPointe NM, Kramer JM, Califf RM (2003) What clinicians should know about the QT interval. JAMA 289: 2120–2127. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous