A dynamic microtubule cytoskeleton directs medial actomyosin function during tube formation
- PMID: 24914560
- PMCID: PMC4064686
- DOI: 10.1016/j.devcel.2014.03.023
A dynamic microtubule cytoskeleton directs medial actomyosin function during tube formation
Abstract
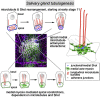
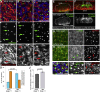
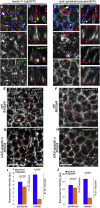
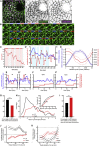
The cytoskeleton is a major determinant of cell-shape changes that drive the formation of complex tissues during development. Important roles for actomyosin during tissue morphogenesis have been identified, but the role of the microtubule cytoskeleton is less clear. Here, we show that during tubulogenesis of the salivary glands in the fly embryo, the microtubule cytoskeleton undergoes major rearrangements, including a 90° change in alignment relative to the apicobasal axis, loss of centrosomal attachment, and apical stabilization. Disruption of the microtubule cytoskeleton leads to failure of apical constriction in placodal cells fated to invaginate. We show that this failure is due to loss of an apical medial actomyosin network whose pulsatile behavior in wild-type embryos drives the apical constriction of the cells. The medial actomyosin network interacts with the minus ends of acentrosomal microtubule bundles through the cytolinker protein Shot, and disruption of Shot also impairs apical constriction.
Copyright © 2014 The Authors. Published by Elsevier Inc. All rights reserved.
Figures



Similar articles
-
β-H-Spectrin is a key component of an apical-medial hub of proteins during cell wedging in tube morphogenesis.J Cell Sci. 2024 Aug 1;137(15):jcs261946. doi: 10.1242/jcs.261946. Epub 2024 Aug 12. J Cell Sci. 2024. PMID: 38988298 Free PMC article.
-
A release-and-capture mechanism generates an essential non-centrosomal microtubule array during tube budding.Nat Commun. 2021 Jul 2;12(1):4096. doi: 10.1038/s41467-021-24332-0. Nat Commun. 2021. PMID: 34215746 Free PMC article.
-
A contractile actomyosin network linked to adherens junctions by Canoe/afadin helps drive convergent extension.Mol Biol Cell. 2011 Jul 15;22(14):2491-508. doi: 10.1091/mbc.E11-05-0411. Epub 2011 May 25. Mol Biol Cell. 2011. PMID: 21613546 Free PMC article.
-
Epithelial polarity and morphogenesis.Curr Opin Cell Biol. 2011 Oct;23(5):540-6. doi: 10.1016/j.ceb.2011.07.005. Epub 2011 Jul 30. Curr Opin Cell Biol. 2011. PMID: 21807488 Review.
-
Microtubules enter centre stage for morphogenesis.Philos Trans R Soc Lond B Biol Sci. 2020 Oct 12;375(1809):20190557. doi: 10.1098/rstb.2019.0557. Epub 2020 Aug 24. Philos Trans R Soc Lond B Biol Sci. 2020. PMID: 32829681 Free PMC article. Review.
Cited by
-
Stable Force Balance between Epithelial Cells Arises from F-Actin Turnover.Dev Cell. 2015 Dec 21;35(6):685-97. doi: 10.1016/j.devcel.2015.11.018. Epub 2015 Dec 10. Dev Cell. 2015. PMID: 26688336 Free PMC article.
-
A role for endoplasmic reticulum dynamics in the cellular distribution of microtubules.Proc Natl Acad Sci U S A. 2022 Apr 12;119(15):e2104309119. doi: 10.1073/pnas.2104309119. Epub 2022 Apr 4. Proc Natl Acad Sci U S A. 2022. PMID: 35377783 Free PMC article.
-
Uncoupling apical constriction from tissue invagination.Elife. 2017 Mar 6;6:e22235. doi: 10.7554/eLife.22235. Elife. 2017. PMID: 28263180 Free PMC article.
-
Mechanical state transitions in the regulation of tissue form and function.Nat Rev Mol Cell Biol. 2024 Aug;25(8):654-670. doi: 10.1038/s41580-024-00719-x. Epub 2024 Apr 10. Nat Rev Mol Cell Biol. 2024. PMID: 38600372 Review.
-
Orchestrating morphogenesis: building the body plan by cell shape changes and movements.Development. 2020 Sep 11;147(17):dev191049. doi: 10.1242/dev.191049. Development. 2020. PMID: 32917667 Free PMC article. Review.
References
-
- Akhmanova A., Stehbens S.J., Yap A.S. Touch, grasp, deliver and control: functional cross-talk between microtubules and cell adhesions. Traffic. 2009;10:268–274. - PubMed
-
- Bartolini F., Gundersen G.G. Generation of noncentrosomal microtubule arrays. J. Cell Sci. 2006;119:4155–4163. - PubMed
-
- Basu R., Chang F. Shaping the actin cytoskeleton using microtubule tips. Curr. Opin. Cell Biol. 2007;19:88–94. - PubMed
-
- Bertet C., Sulak L., Lecuit T. Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature. 2004;429:667–671. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- BB/B501798/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- MC_U105178780/MRC_/Medical Research Council/United Kingdom
- BB/J010278/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- U105178780/MRC_/Medical Research Council/United Kingdom
- WT090279MA/WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

