Reduced inflammatory phenotype in microglia derived from neonatal rat spinal cord versus brain
- PMID: 24914808
- PMCID: PMC4051776
- DOI: 10.1371/journal.pone.0099443
Reduced inflammatory phenotype in microglia derived from neonatal rat spinal cord versus brain
Abstract
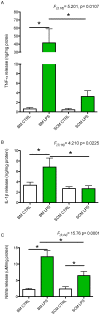
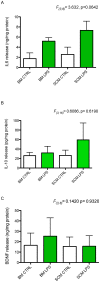
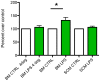
Microglia are the primary immune cells of the central nervous system (CNS). Membrane bound sensors on their processes monitor the extracellular environment and respond to perturbations of the CNS such as injury or infection. Once activated, microglia play a crucial role in determining neuronal survival. Recent studies suggest that microglial functional response properties vary across different regions of the CNS. However, the activation profiles of microglia derived from the spinal cord have not been evaluated against brain microglia in vitro. Here, we studied the morphological properties and secretion of inflammatory and trophic effectors by microglia derived from the brain or spinal cord of neonatal rats under basal culture conditions and after activation with lipopolysaccharide (LPS). Our results demonstrate that spinal microglia assume a less inflammatory phenotype after LPS activation, with reduced release of the inflammatory effectors tumor necrosis factor alpha, interleukin-1 beta, and nitric oxide, a less amoeboid morphology, and reduced phagocytosis relative to brain-derived microglia. Phenotypic differences between brain and spinal microglia are an important consideration when evaluating anti-inflammatory or immunomodulatory therapies for brain versus spinal injury.
Conflict of interest statement
Figures


References
-
- Rezaie P, Male D (1999) Colonisation of the developing human brain and spinal cord by microglia: a review. Microscopy research and technique 45: 359–382. - PubMed
-
- Aloisi F (2001) Immune function of microglia. Glia 36: 165–179. - PubMed
-
- Lai AY, Todd KG (2006) Hypoxia-activated microglial mediators of neuronal survival are differentially regulated by tetracyclines. Glia 53: 809–816. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

