Hypothalamic molecular changes underlying natural reproductive senescence in the female rat
- PMID: 24914937
- PMCID: PMC4138577
- DOI: 10.1210/en.2014-1017
Hypothalamic molecular changes underlying natural reproductive senescence in the female rat
Abstract
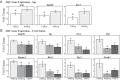
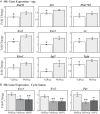
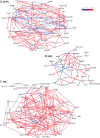
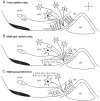
The role of the hypothalamus in female reproductive senescence is unclear. Here we identified novel molecular neuroendocrine changes during the natural progression from regular reproductive cycles to acyclicity in middle-aged female rats, comparable with the perimenopausal progression in women. Expression of 48 neuroendocrine genes was quantified within three hypothalamic regions: the anteroventral periventricular nucleus, the site of steroid positive feedback onto GnRH neurons; the arcuate nucleus (ARC), the site of negative feedback and pulsatile GnRH release; and the median eminence (ME), the site of GnRH secretion. Surprisingly, the majority of changes occurred in the ARC and ME, with few effects in anteroventral periventricular nucleus. The overall pattern was increased mRNA levels with chronological age and decreases with reproductive cycle status in middle-aged rats. Affected genes included transcription factors (Stat5b, Arnt, Ahr), sex steroid hormone receptors (Esr1, Esr2, Pgr, Ar), steroidogenic enzymes (Sts, Hsd17b8), growth factors (Igf1, Tgfa), and neuropeptides (Kiss1, Tac2, Gnrh1). Bionetwork analysis revealed region-specific correlations between genes and hormones. Immunohistochemical analyses of kisspeptin and estrogen receptor-α in the ARC demonstrated age-related decreases in kisspeptin cell numbers as well as kisspeptin-estrogen receptor-α dual-labeled cells. Taken together, these results identify unexpectedly strong roles for the ME and ARC during reproductive decline and highlight fundamental differences between middle-aged rats with regular cycles and all other groups. Our data provide evidence of decreased excitatory stimulation and altered hormone feedback with aging and suggest novel neuroendocrine pathways that warrant future study. Furthermore, these changes may impact other neuroendocrine systems that undergo functional declines with age.
Figures


Similar articles
-
Sex steroid control of hypothalamic Kiss1 expression in sheep and rodents: comparative aspects.Peptides. 2009 Jan;30(1):94-102. doi: 10.1016/j.peptides.2008.08.013. Epub 2008 Aug 26. Peptides. 2009. PMID: 18789989 Review.
-
IGF1 gene therapy in middle-aged female rats delays reproductive senescence through its effects on hypothalamic GnRH and kisspeptin neurons.Aging (Albany NY). 2022 Nov 1;14(21):8615-8632. doi: 10.18632/aging.204360. Epub 2022 Nov 1. Aging (Albany NY). 2022. PMID: 36326686 Free PMC article.
-
Reduced responsiveness of kisspeptin neurons to estrogenic positive feedback associated with age-related disappearance of LH surge in middle-age female rats.Gen Comp Endocrinol. 2013 Nov 1;193:121-9. doi: 10.1016/j.ygcen.2013.06.024. Epub 2013 Jul 10. Gen Comp Endocrinol. 2013. PMID: 23851104
-
Disruption of reproductive aging in female and male rats by gestational exposure to estrogenic endocrine disruptors.Endocrinology. 2013 Jun;154(6):2129-43. doi: 10.1210/en.2012-2123. Epub 2013 Apr 16. Endocrinology. 2013. PMID: 23592748 Free PMC article.
-
Kisspeptin signalling in the brain: steroid regulation in the rodent and ewe.Brain Res Rev. 2008 Mar;57(2):288-98. doi: 10.1016/j.brainresrev.2007.04.002. Epub 2007 Apr 19. Brain Res Rev. 2008. PMID: 17509691 Review.
Cited by
-
1,3-Dicaffeoylquinic Acid as an Active Compound of Arctium lappa Root Extract Ameliorates Depressive-Like Behavior by Regulating Hippocampal Nitric Oxide Synthesis in Ovariectomized Mice.Antioxidants (Basel). 2021 Aug 12;10(8):1281. doi: 10.3390/antiox10081281. Antioxidants (Basel). 2021. PMID: 34439529 Free PMC article.
-
Effects of endocrine-disrupting chemicals on hypothalamic oxytocin and vasopressin systems.J Exp Zool A Ecol Integr Physiol. 2022 Jan;337(1):75-87. doi: 10.1002/jez.2475. Epub 2021 May 21. J Exp Zool A Ecol Integr Physiol. 2022. PMID: 34018699 Free PMC article.
-
Decreased Anti-Müllerian hormone and Anti-Müllerian hormone receptor type 2 in hypothalami of old Japanese Black cows.J Vet Med Sci. 2020 Aug 19;82(8):1113-1117. doi: 10.1292/jvms.20-0159. Epub 2020 Jun 17. J Vet Med Sci. 2020. PMID: 32554955 Free PMC article.
-
Restoring neuropetide Y levels in the hypothalamus ameliorates premature aging phenotype in mice.Geroscience. 2025 Feb 27. doi: 10.1007/s11357-025-01574-0. Online ahead of print. Geroscience. 2025. PMID: 40011349
-
The Methylcytosine Dioxygenase Ten-Eleven Translocase-2 (tet2) Enables Elevated GnRH Gene Expression and Maintenance of Male Reproductive Function.Endocrinology. 2016 Sep;157(9):3588-603. doi: 10.1210/en.2016-1087. Epub 2016 Jul 6. Endocrinology. 2016. PMID: 27384303 Free PMC article.
References
-
- Scarbrough K, Wise PM. Age-related changes in pulsatile luteinizing hormone release precede the transition to estrous acyclicity and depend upon estrous cycle history. Endocrinology. 1990;126:884–890 - PubMed
-
- Matt DW, Gilson MP, Sales TE, et al. Characterization of attenuated proestrous luteinizing hormone surges in middle-aged rats by deconvolution analysis. Biol Reprod. 1998;59:1477–1482 - PubMed
-
- Zuo Z, Mahesh VB, Zamorano PL, Brann DW. Decreased gonadotropin-releasing hormone neurosecretory response to glutamate agonists in middle-aged female rats on proestrus afternoon: a possible role in reproductive aging? Endocrinology. 1996;137:2334–2338 - PubMed
-
- Gore AC, Oung T, Yung S, Flagg RA, Woller MJ. Neuroendocrine mechanisms for reproductive senescence in the female rat: gonadotropin-releasing hormone neurons. Endocrine. 2000;13:315–323 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

