Protein expression, characterization, crystallization and preliminary X-ray crystallographic analysis of a Fic protein from Clostridium difficile
- PMID: 24915103
- PMCID: PMC4051547
- DOI: 10.1107/S2053230X1400987X
Protein expression, characterization, crystallization and preliminary X-ray crystallographic analysis of a Fic protein from Clostridium difficile
Abstract
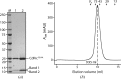
Fic domains in proteins are found in abundance in nature from the simplest prokaryotes to animals. Interestingly, Fic domains found in two virulence factors of Gram-negative bacteria have recently been demonstrated to catalyse the transfer of the AMP moiety from ATP to small host GTPases. This post-translational modification has attracted considerable interest and a role for adenylylation in pathology and physiology is emerging. This work was aimed at the structural characterization of a newly identified Fic protein of the Gram-positive bacterium Clostridium difficile. A constitutively active inhibitory helix mutant of C. difficile Fic was overexpressed in Escherichia coli, purified and crystallized by the vapour-diffusion technique. Preliminary X-ray crystallographic analysis shows that the crystals diffract to at least 1.68 Å resolution at a synchrotron X-ray source. The crystals belonged to the orthorhombic space group P2₁2₁2₁, with unit-cell parameters a=45.6, b=80.8, c=144.7 Å, α=β=γ=90°. Two molecules per asymmetric unit corresponds to a Matthews coefficient of 2.37 Å3 Da(-1) and a solvent content of 48%.
Keywords: Clostridium difficile; Fic protein; adenylylation.
Figures


References
-
- Compton, L. A. & Johnson, W. C. Jr (1986). Anal. Biochem. 155, 155–167. - PubMed
-
- Diederichs, K. & Karplus, P. A. (1997). Nature Struct. Biol. 4, 269–275. - PubMed
-
- Engel, P., Goepfert, A., Stanger, F. V., Harms, A., Schmidt, A., Schirmer, T. & Dehio, C. (2012). Nature (London), 482, 107–110. - PubMed
-
- Faber, P. W., Barnes, G. T., Srinidhi, J., Chen, J., Gusella, J. F. & MacDonald, M. E. (1998). Hum. Mol. Genet. 7, 1463–1474. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

