Generation of three-dimensional retinal tissue with functional photoreceptors from human iPSCs
- PMID: 24915161
- PMCID: PMC4370190
- DOI: 10.1038/ncomms5047
Generation of three-dimensional retinal tissue with functional photoreceptors from human iPSCs
Abstract
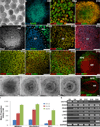
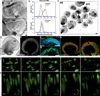
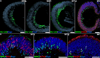
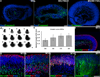
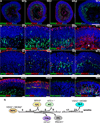
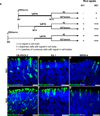
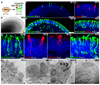
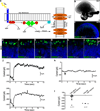
Many forms of blindness result from the dysfunction or loss of retinal photoreceptors. Induced pluripotent stem cells (iPSCs) hold great potential for the modelling of these diseases or as potential therapeutic agents. However, to fulfill this promise, a remaining challenge is to induce human iPSC to recreate in vitro key structural and functional features of the native retina, in particular the presence of photoreceptors with outer-segment discs and light sensitivity. Here we report that hiPSC can, in a highly autonomous manner, recapitulate spatiotemporally each of the main steps of retinal development observed in vivo and form three-dimensional retinal cups that contain all major retinal cell types arranged in their proper layers. Moreover, the photoreceptors in our hiPSC-derived retinal tissue achieve advanced maturation, showing the beginning of outer-segment disc formation and photosensitivity. This success brings us one step closer to the anticipated use of hiPSC for disease modelling and open possibilities for future therapies.
Conflict of interest statement
Under a licensing agreement between Life Technologies Corporation and the Johns Hopkins University (JHU), E.T.Z. is entitled to a share of royalty received by the University for licensing of stem cells. The terms of this arrangement are managed by JHU in accordance with its Conflict of Interest policies. This does not alter authors’ adherence to journal policies on sharing data and materials. Requests for CB-iPSC6.2 and KA.1 cell lines should be addressed to E.T.Z. (
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

